Fetal brain anomalies have a wide differential diagnosis including: (1) congenital genetic and vascular disorders; (2) acquired causes such as infection and hypoxic-ischemic injury; and rarely (3) neoplasm (Jansen and Keymolen 2019). Fetal brain anomalies are seen infrequently. Consequently, there is limited literature describing methods of distinguishing these conditions, barring the presence of the most characteristic findings on imaging.
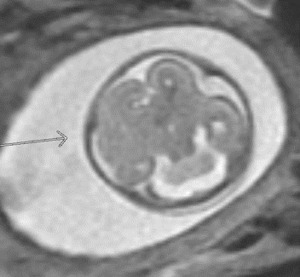
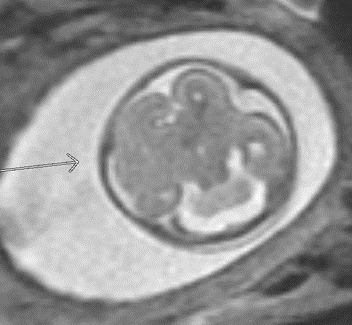
A 24-year-old gravida 1 with a dichorionic-diamniotic twin pregnancy at 16w1d gestation was evaluated by Maternal-Fetal Medicine who identified multiple possible cranio-facial abnormalities in Twin A on transabdominal ultrasound. This raised the concern for a possible chromosomal abnormality as well as holoprosencephaly as the cavum septum pellucidum was not visualized. A detailed transabdominal ultrasound performed at 16w1d provided the initial assessment of the abnormal-appearing left cerebrum of Twin A (Figure 1). The left cerebral ventricle was overall poorly visualized, however, the posterior aspect of the left ventricle appeared to be significantly more dilated compared to the normal-appearing right ventricle. The cavum septum pellucidum could not be visualized, the thalami appeared fused, and the temporal areas of the facies appeared more angulated than expected. The patient was scheduled for a fetal MRI which was performed at 19w1d gestation and showed complete architectural distortion involving the left cerebral hemisphere of Twin A, with a mass-like structure measuring approximately 2.4 x 2.3 x 1.8 cm, along with a loss of normal gray-white differentiation (Figure 2). The diagnoses based on this MRI were either a neoplastic growth or a migrational anomaly.
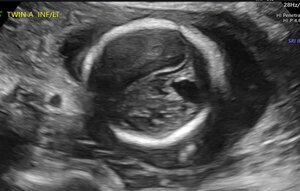
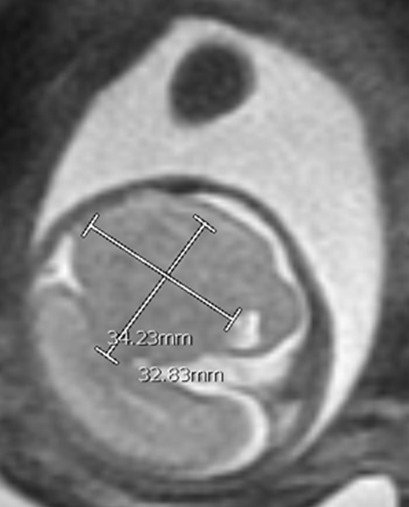
At 20w0d gestation, the patient returned for a repeat ultrasound study which showed a left cerebral mass with a dysplastic appearance, and evident mass effect on the ipsilateral ventricle (Figure 3). The expanded differential at that time included a fetal brain mass, with the highest suspicion for a teratoma. Less likely etiologies included a malformation secondary to a cerebral vascular accident and a congenital brain malformation. As no in utero interventions were indicated based on the suspected diagnosis, focus was directed toward confirming the presence of a fetal brain tumor. Relative growth of this suspected mass over time, measured by a repeat fetal MRI, was possibly suggestive of an aggressive fetal neoplasm.
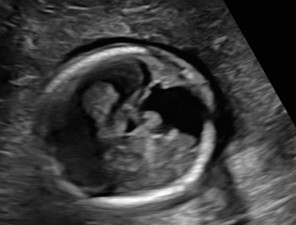
At 23w0d a limited fetal MRI was repeated (Figure 4). This study demonstrated, again, the supratentorial left hemispheric mass measuring 3.3 x 3.5 x 2.4 cm within the cerebrum of Twin A. Most notably, there was an interval increase in the size of the mass relative to the skull diameter, as well as a prominent mass effect on the contralateral hemisphere with an associated midline shift. This second fetal MRI finding was interpreted as most consistent with an enlarging intracranial neoplasm with the differential diagnoses including astrocytoma, intracranial teratoma, and glioblastoma multiforme; however, no distinctive features were noted to confirm one of these diagnoses. The interval growth of the mass was interpreted as characteristic of an aggressive fetal neoplasm; therefore, a migrational anomaly was seen as less likely.
Anticipating the scope of Twin A’s neonatal care needs, it was recommended that the patient deliver at a center with neuro-oncology services and a robust neonatal support center. The patient was transferred to Children’s Hospital Los Angeles for further care. At 30w5d, a prenatal ultrasound study revealed an echogenic nonvascular intracranial mass in the left hemisphere measuring 4.18 x 3.57 cm with significant mass effect along with ventricular dilation. A repeat fetal MRI at 32w0d gestation confirmed a markedly abnormal mass-like configuration in the left cerebral hemisphere and absence of a cava septum pellucidum, basal ganglia, corpus callosum, and right ventricle. Additionally, this MRI had findings suggestive of extensive migrational abnormalities throughout the left cerebral hemisphere, best seen superiorly. It noted no solid or cystic masses and no areas of abnormally restricted diffusion or hemorrhage. While a congenital brain tumor could not be ruled-out, this MRI study reordered the differential diagnosis that had previously favored fetal brain neoplasm.
The patient continued her obstetric care at the Children’s Hospital Los Angeles, and at 35w3d, presented with preterm prelabor rupture of membranes. Both infants were delivered via cesarean section without complication.
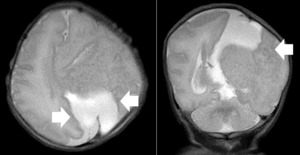
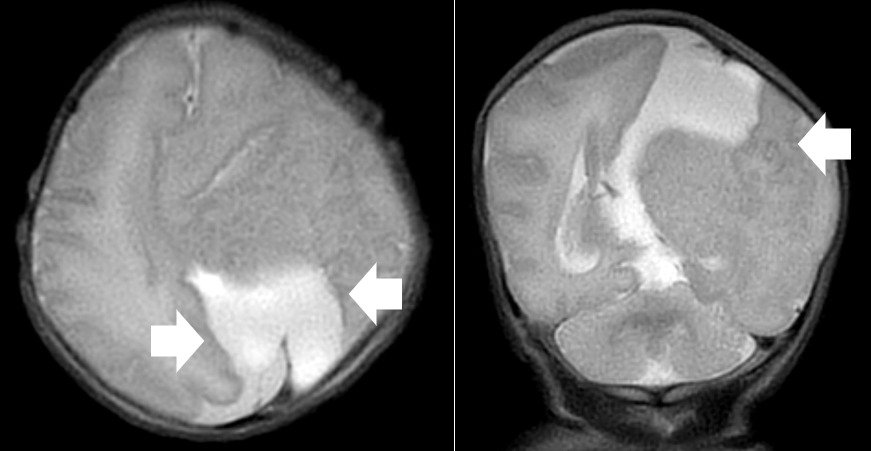
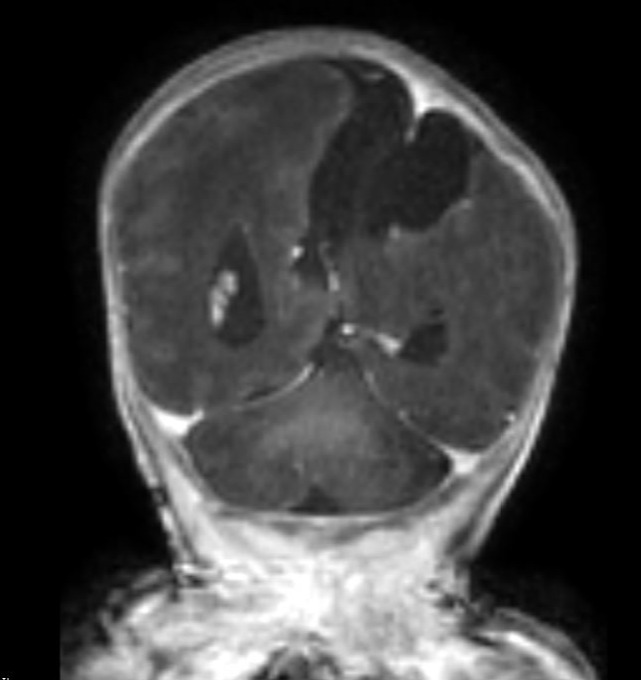
A repeat MRI on day-of-life 1 revealed multiple intracranial anomalies including extensive polymicrogyria of the left cerebral hemisphere, polymicrogyria in the right frontal lobe, incomplete development of the left lateral ventricle, and a gray matter lined cerebrospinal fluid cleft in the left posterior-frontal and parietal regions communicating with the rudimentary left lateral ventricle (Figures 5-7). The dysplastic left cerebral hemisphere exerted a mass effect on the rudimentary left lateral ventricle with a left-to-right midline shift measuring approximately 6 mm at the level of the foramen of Monroe. There was evidence of partial fusion of the frontal lobes. In summary, the first postnatal MRI confirmed that rather than a growing neoplasm, Twin A had extensive intracranial developmental anomalies, including a dysplastic left cerebral cortex, schizencephaly, and hypoplasia or absence of multiple midline structures.
Twin A received NICU care at Children’s Hospital of Los Angeles until 5w5d of life, when the infant was discharged home. The neonate’s cranial findings remained stable throughout his NICU stay. Per the Neurology team, his prognosis included developmental delays with a high risk of a severe seizure disorder, an oxygen requirement, and difficulty with feeding, requiring placement of a gastrostomy tube.
DISCUSSION
Antenatal diagnosis of fetal brain anomalies is inherently complex. First, there is a broad differential for brain anomalies which is generally divided into genetic and acquired causes. Disorders of a genetic origin include congenital malformations of both the brain and the spine, as well as metabolic and vascular disorders that can affect normal brain development (Jansen and Keymolen 2019). Acquired causes include infections, hypoxic-ischemic insults, traumatic brain injury, and a number of rare neoplasms (Jansen and Keymolen 2019). We discuss a case where an early fetal brain abnormality had multiple findings mimicking a growing neoplasm. However, serial Ultrasounds and MRIs across multiple institutions ultimately revealed a migrational brain abnormality late in the prenatal course.
Both fetal neoplasms and migratory abnormalities are exceedingly rare (Cavalheiro et al. 2003). Fetal neoplasms are seen in only 0.34 per million live births (Stiller and Bunch 1992), and typically require repeat MRI studies throughout the prenatal period to identify distinctive characteristics for formal diagnosis. The diagnosis of fetal neoplasms can be problematic for several reasons. Their prevalence, location, and behaviors differ when compared to pediatric intracranial neoplasms (Kline-Fath, Bulas, and Lee 2020). Fetal tumors grow rapidly and will reach large proportions due to the high baseline mitotic rate seen in embryonic cells. These tumors are typically supratentorial, and their epicenter can be difficult to locate due to their size and extent of the neoplasm (Pilu, Malinger, and Buyukkurt 2013; Fernández-Mayoralas et al. 2010).
On the other hand, migrational disorders are more common than fetal brain tumors in singleton gestations, and even more so in multi-gestational pregnancies (Kline-Fath, Bulas, and Lee 2020). Migrational disorders encompass a wide variety of diagnoses. Both the rarity of these conditions and the necessity of repeat imaging with detailed interpretation naturally limits the available literature on how to distinguish between these two pathologies. As imaging findings developed throughout this case, the key migrational anomaly we considered was schizencephaly, a disorder characterized by congential full-thickness grey matter-lined clefts of the cerebral mantle (Pilu, Malinger, and Buyukkurt 2013). A look into available literature clearly shows the difficulty in making this diagnosis prenataly. Thus far, prenatal diagnosis has only been reported for cases with widely open clefts (Fernández-Mayoralas et al. 2010; Hung et al. 2008) otherwise known as Type II or open-lipped schizencephaly. In these cases, early ultrasound studies and fetal MRIs have shown the presence of fluid filled clefts connecting the lateral ventricle and subarachnoid spaces (Fernández-Mayoralas et al. 2010; Hung et al. 2008). However, Type 1 schizencephaly with small, fused defects have not been identified antenatally and it is uncertain whether it would even be possible to make a certain diagnosis with CT or MRI until after delivery (Pilu, Malinger, and Buyukkurt 2013; Hung et al. 2008; Woodward et al. 2005; Schlembach et al. 1999).
There are several reasons why schizencephaly is easily not diagnosed in the early gestational period (Schlembach et al. 1999; Curry et al. 2005a). The pathologic process which precedes schizencephaly is likely to occur before 24 weeks gestation (Howe, Rankin, and Draper 2011). However, the characteristic imaging finding for Type 2 defects, a gray matter-lined cleft extending from the ventricular surface to the subarachnoid space, only evolves later in the gestation and is identified most commonly after 28 weeks (Barkovich and Kjos 1992). While it is assusmed that type 1 defects should form around this gestational age, the defect at this stage is so small and difficult to visualize that there are no case reports of this diagnosed before delivery, and no consensus whether increased US, CT, or MRI screening would be able to definitively identify such slight findings until after delivery (Pilu, Malinger, and Buyukkurt 2013; Hung et al. 2008; Woodward et al. 2005; Schlembach et al. 1999).
Therefore, our case is a key example that shows observation of a dysplastic-appearing, mass-like region, increasing in size throughout pregnancy, necessitates consideration of fetal migrational disorders. MRI is the gold standard for diagnosing fetal brain abnormalities; however, even with repeat imaging studies over months, there is significant difficulty in distinguishing between fetal brain neoplasms and migrational anomalies. Interpretation of our initial MRI at 19w1d gestation did not clearly favor either disorder. However, the repeat MRI at 23w0d gestation demonstrated an increase in the size of the mass relative to the rest of the skull and was interpreted using conventional analysis as characteristic of an enlarging fetal neoplasm. It was not until a third MRI at 32w0d gestation that migrational anomaly was considered the leading diagnosis. Furthermore, a final diagnosis of schizencephaly itself was not specifically commented on until the postnatal period. Despite our difficulties making the correct diagnosis, certain elements in the patients’ history also suggested an increased likelihood of migrational anomalies.
It is well established that twins have an increased risk for congenital malformations and disruptions. In particular, polymicrogyria and schizencephaly are observed more commonly in twin pregnancies than in singleton gestations (Park et al. 2021; Curry et al. 2005b). While the exact prevalence of schizencephaly in twin pregnancies is not known, Park et al. noted schizencephaly in 22% of twin pregnancies which were complicated by malformations of cortical development (Park et al. 2021). In contrast, no increased risk in neonatal brain neoplasms has been cited in twin gestations. Therefore, in future cases, we recommend a high suspicion for migrational disorders when noting supratentorial brain anomalies in twin gestations.
TEACHING POINT
Features used to diagnose fetal brain anomalies are often nonspecific and develop throughout a gestation. Most diagnoses require multiple imaging studies over time to identify, and key features that are characteristic often do not present until very late in a gestation. The differential diagnosis of a dysplastic appearing mass-like region growing in size throughout a pregnancy should include fetal migrational disorders.
Authors Contributions
Eric Michael Schmitt: Primary Author. Chiefly responsible for concept and design of manuscript.
Pablo Antonio Delis: Made substantial contributions to analysis and interpretation of data.
Sonia Pinto: Made substantial contributions to analysis and interpretation of data, especially in acquiring and analyzing fetal images from Children’s Hospital of Los Angeles.
Veronica Rooks: Made substantial contributions to analysis and interpretation of data, especially in acquiring and analyzing fetal images from Tripler Army Medical Center.
Donald Jay Gloeb: Senior Author. Made substantial contributions to analysis and interpretation of data, especially in acquiring and analyzing fetal images from Tripler Army Medical Center.
Disclosures
The Author(s) reports no conflicts of interest, and no funding has been received for this work.
This case report has been presented at ACOG Armed Forces District Conference 2022; 2022 October 16-19; Henderson, Nevada.
The views expressed herein are those of the presenter and do not reflect the official policy of the Department of the Army, Department of Defense, Defense Health Agency, or U.S. Government.
Disclaimer
Eric M SCHMITT, MD, Pablo A DELIS, MD, Veronica ROOKS, MD, and Donald J GLOEB, DO are employed by the Armed Forces.