Introduction
EP represents a critical obstetric emergency where the fertilized ovum implants outside the endometrial cavity, most frequently in the ampullary segment of the fallopian tube. Though relatively infrequent, occurring in approximately 1–2% of all gestations, it remains a significant contributor to early pregnancy-related maternal morbidity, particularly during the first trimester (“ACOG Practice Bulletin No. 193: Tubal Ectopic Pregnancy” 2018). Management often necessitates surgical intervention, with salpingectomy being a common approach in tubal cases. Following removal, serum beta-human chorionic gonadotropin (β-hCG) levels are expected to decline steadily. Deviations from this expected decline pattern warrant thorough investigation.
Persistently elevated or rising β-hCG after surgery may signal several potential complications. One common explanation is the incomplete excision of trophoblastic tissue, especially following conservative procedures such as salpingostomy, where tissue remnants may continue to produce β-hCG. Alternatively, persistent ectopic gestation, characterized by the ongoing proliferation of viable ectopic trophoblasts, must be considered (van Mello et al. 2012). A less frequent but critical differential is gestational trophoblastic neoplasia, including choriocarcinoma, which typically manifests with aggressive β-hCG elevation and systemic signs (Seckl, Sebire, and Berkowitz 2010). Additionally, HP, the concurrent presence of both intrauterine and ectopic pregnancies, can also account for residual or rising β-hCG (Barrenetxea et al. 2007).
Though heterotopic gestation is rare in spontaneous pregnancies (approximately 1 in 30,000), its prevalence significantly increases in patients undergoing assisted reproductive techniques (ART), reaching an estimated incidence of 1 in 100 cycles (Wang et al. 2023). Risk factors for HP mirror those for EP and are largely iatrogenic in ART contexts. These include mechanical and technical variables such as the number and site of embryo transfers, embryo quality, the hormonal environment at transfer, and the volume of transfer medium. Additionally, anatomic disruptions due to endometriosis, pelvic inflammatory disease, or prior surgery can contribute to abnormal implantation (Felekis et al. 2014).
Case Report
A 27-year-old gravida 2, para 1 woman with a spontaneously conceive pregnancy at 6 weeks gestation presented to the Emergency Department with acute left lower quadrant abdominal pain and mild vaginal spotting. Quantitative serum β-hCG measured 7,539 mIU/mL. Transabdominal ultrasound failed to identify an intrauterine pregnancy but revealed the presence of free fluid in the pelvis and a small intrauterine pseudosac. Speculum examination demonstrated a normal cervix with scant vaginal bleeding.
Diagnostic laparoscopy revealed a normal-appearing uterus and ovaries. However, the left fallopian tube was noted to be dilated and consistent with an EP. Approximately 100 mL of hemoperitoneum was evacuated, and a left salpingectomy was performed. Pathologic examination of the resected fallopian tube confirmed the diagnosis of a tubal ectopic pregnancy with hemorrhage and occasional trophoblasts, compatible with tubal (ectopic) pregnancy. No hydropic structures or fetal somatic tissues were identified. These findings were consistent with a tubal ectopic pregnancy and ruled out the possibility of a hydrosalpinx.
The patient did not receive follow-up β-hCG monitoring postoperatively. Four weeks after surgery, she obtained a positive home pregnancy test. Upon evaluation in clinic, the patient was counseled that a new intrauterine pregnancy was unlikely, and the test result was presumed to reflect residual trophoblastic tissue. A serum β-hCG at that time was significantly elevated at 51,414 mIU/mL.
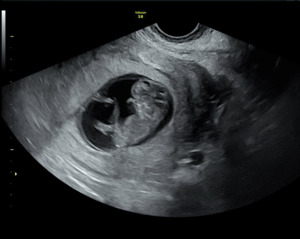
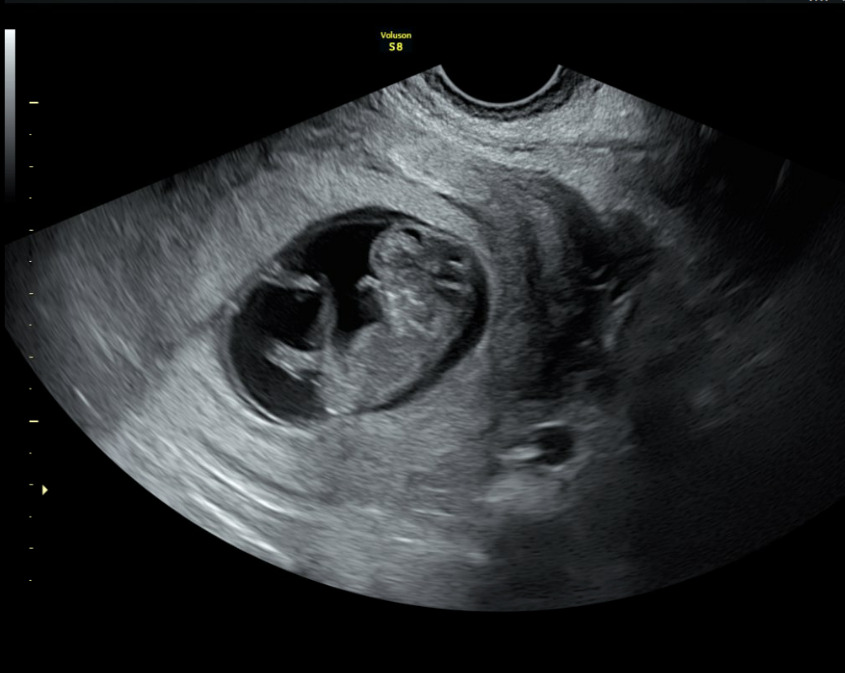
A formal transabdominal and transvaginal ultrasound subsequently revealed a viable intrauterine pregnancy consistent with 10 weeks gestation, confirming the diagnosis of a missed intrauterine pregnancy coexisting with a resolved EP: a HP. The fetus demonstrated appropriate growth and viability. The patient elected to continue the pregnancy and initiated prenatal care.
A fetal anatomy scan performed at 21 weeks gestation demonstrated a single live intrauterine fetus with biometric measurements consistent with normal growth. Detailed evaluation revealed no gross structural abnormalities in the abdomen, three-vessel umbilical cord with normal insertion, kidneys, stomach, bladder, spine, extremities, or face. Intracranial anatomy, including the choroid plexus, lateral ventricles, cerebellum, and cisterna magna, appeared normal. A screening fetal echocardiogram was also within normal limits, with appropriate fetal movements observed throughout the examination.
Discussion
HP is a rare and often overlooked cause of persistent β-hCG after EP removal. The condition presents a diagnostic challenge due to its rarity and clinical overlap with more common obstetric conditions. A substantial proportion of HP cases are asymptomatic (about 50%) until rupture occurs, which often prompts emergency presentation. Reports suggest that up to 78.5% of HPs are diagnosed only after the rupture of the ectopic component, typically the fallopian tube, manifesting as an acute abdomen and hemodynamic instability (Felekis et al. 2014). Such presentations can be indistinguishable from isolated ectopic pregnancies or complicated miscarriages, leading to diagnostic delays. Pelvic pain, vaginal bleeding, and signs of peritoneal irritation are not unique to HP and can be misleading, especially when clinicians are not actively considering the possibility of dual gestation (Mohapatra and Samantaray 2022). The limitations of β-hCG monitoring further complicate diagnosis. In normal early pregnancy, β-hCG levels rise predictably, but in the context of HP, a viable intrauterine pregnancy can mask the continued trophoblastic activity of the ectopic gestation. As a result, serum β-hCG levels may rise appropriately, misleading clinicians into presuming a reassuring trajectory. This is particularly problematic when surgical treatment of the EP is presumed complete, and subsequent β-hCG monitoring fails to show the expected decline. β-hCG is a marker of hormonal activity rather than gestational location, making it inherently limited as a diagnostic tool in such scenarios (Felekis et al. 2014). Ultrasound imaging, while central to early pregnancy evaluation, is also susceptible to misinterpretation. Early ectopic pregnancies may appear sonographically similar to other adnexal findings such as hemorrhagic corpus luteum cysts, tubo-ovarian masses, or benign neoplasms, thus increasing the complexity in cases with overlapping pathologies. Such diagnostic ambiguity is especially pronounced in natural conceptions, where suspicion of HP is inherently lower, resulting in delayed diagnosis (Sun et al. 2012).
A thorough diagnostic and post-operative workup are essential in managing ectopic pregnancies in the case of HP to avoid missing other differentials such as trophoblastic tissue remnants. These tissues can penetrate local structures, making their complete removal particularly challenging in conservative approaches such as salpingostomy. Even when the visible ectopic mass is excised, microscopic remnants may remain embedded in the fallopian tube or surrounding peritoneum. This residual tissue can continue to grow or bleed, causing ongoing or delayed internal hemorrhage, hemoperitoneum, or secondary rupture. Additionally, such remnants may lead to localized infection or inflammatory responses, complicating both the clinical picture and recovery. These complications can delay the diagnosis of persistent EP and interrupt timely decision-making in fertility planning. When β-hCG levels fail to decline as expected post-surgery clinical uncertainty may arise, sometimes requiring additional interventions such as methotrexate therapy or a second surgical procedure (Alkatout et al. 2017). Although less common, gestational trophoblastic neoplasia must be considered in any reproductive-aged patient with persistently elevated or rapidly rising β-hCG levels following pregnancy-related events, including EP, miscarriage, molar pregnancy, or elective abortion. This neoplastic process is characterized by its aggressive nature and potential for systemic dissemination, particularly to the lungs, liver, or brain (Meyers et al. 2025). Delayed recognition can result in advanced disease progression, underscoring the need for early exclusion through serial β-hCG monitoring and imaging when appropriate.
Finally, the possibility of HP should not be overlooked when β-hCG trends deviate from expected norms. The discovery of a viable intrauterine pregnancy has critical implications. Early recognition allows for the initiation of prenatal care, including folic acid supplementation, screening for congenital anomalies, and management of maternal conditions such as hypertension or diabetes (Cucinella et al., n.d.). Failure to detect a viable intrauterine pregnancy promptly can delay these interventions and potentially compromise maternal-fetal outcomes. Conversely, when the intrauterine pregnancy is nonviable, retained products of conception can become infected as tissue remaining may lead to endometritis or systemic sepsis if not promptly addressed (Rouse et al. 2019). Equally important is the psychological dimension of post-pregnancy loss care. Many patients experience profound emotional distress following ectopic or failed pregnancies. The uncertainty that often accompanies such diagnoses can lead to confusion, fear, and long-term emotional consequences. Clear communication regarding reproductive status, whether a pregnancy was intrauterine or ectopic, viable or nonviable, can help patients process the experience, regain a sense of control, and engage in future fertility planning with confidence. Providing this information sensitively fosters trust and psychological healing, reinforcing the importance of comprehensive and compassionate care (Hasani et al. 2021).
Conclusion
HP is a rare but crucial diagnosis to consider when β-hCG levels remain elevated after EP removal. Missing an ongoing intrauterine pregnancy delays essential prenatal care and can endanger maternal-fetal health. This case reinforces the need for systematic follow-up, including β-hCG monitoring, comprehensive imaging, and careful clinical judgment. A multidisciplinary approach improves diagnostic accuracy and patient safety.