INTRODUCTION
It is currently estimated that approximately one in thirty-six children in the United States are diagnosed with autism spectrum disorder (ASD) (Maenner et al. 2023). The etiology of ASD is not clearly understood and is speculated to arise from some single-gene disorders (Jin et al. 2020) and various complex interactions of genetic and environmental factors which result in changes in biochemistry and physiology, and ultimately neurologic function. Some of these changes may be present at birth and influenced by the intrauterine life.
The intrauterine environment and maternal exposures are important in predisposing or protecting the developing fetus from multiple health conditions such as diabetes, obesity, neurologic disorders, and hypertension, to name a few (Simkova, Veleminsky, and Sram 2020; Gómez-Roig et al. 2021). Epigenetics may play a critical role in the impact of exposures on fetal outcomes (Nye et al. 2014; Traglia et al. 2017). The ability to understand the impact and potential associated exposures during pregnancy related to the development of ASD could be crucial in assisting with early diagnoses and providing resources for parents to begin therapies to optimize the child’s development and function. The full scope of the impact of maternal exposures on ASD in the developing offspring is poorly understood.
The objective of this study was to systematically review and chronicle current literature evaluating maternal exposures associated with ASD diagnosis in their offspring.
MATERIALS AND METHODS
We performed a systematic review of the literature, using Ovid MEDLINE databases and reference lists of retrieved articles. Our search included articles published from January 2006 – December 2021 based on the keywords “autistic disorder,” “autism,” “maternal exposure,” “biomarkers” and “pregnancy.” We included articles that were published in English that studied pregnant individuals and the risk of ASD development. We excluded duplicate articles, those that included rodent and invertebrate analyses and all reviews and hypotheses manuscripts. Two authors independently reviewed abstracts found in the initial literature search and obtained full-text manuscripts for abstracts that met inclusion criteria. The full-text manuscripts were then evaluated for inclusion. Any discrepancy of opinion was discussed and settled via a third independent reviewer.
From the included articles, we collected information regarding the study population, gestational period at which the exposure was tested, exposure of interest, methodology for measurement of the exposure and outcome, study results, and study conclusions. We organized the exposures post-hoc into the following categories: biomarkers, environmental exposures, medication exposures, genetic variability, and maternal illnesses and conditions.
Descriptive summaries were presented. Quantitative meta-analyses were not possible due to the nature of the reports and heterogeneity of the measures and outcomes. The project was exempt from IRB approval as not being human subjects research. PRISMA guidelines for systematic review reporting were followed. As we had begun our search and study evaluation before we knew about PROSPERO registration, this study was not eligible for inclusion in PROSPERO per their guidance.
RESULTS
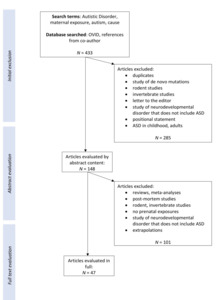
Of 433 reports identified, 47 studies published between 2006 and 2021 fulfilled the inclusion criteria (Figure 1).

Figure 1.Summary of search results and selection of studies for review
This figure summarizes the search outcomes, exclusions, and inclusion
In total, the studies reported on 6,172,363 pregnancies and children ages one to eighteen years with and without ASD. The studies were conducted globally, including 30 in the United States (Traglia et al. 2017; Braun et al. 2014; Granillo et al. 2019; J. H. Kim et al. 2021; Lyall et al. 2017, 2018; McKean et al. 2015; Millenson et al. 2017; Philippat et al. 2018; Ritz et al. 2020; Shin et al. 2018; Geier, Kern, and Geier 2009; Goodrich et al. 2017; Jo et al. 2019; Kalkbrenner et al. 2015; McCanlies et al. 2019; McGuinn et al. 2020; Patti, Li, et al. 2021; Raz et al. 2015; Schmidt et al. 2017; Singer et al. 2016; Vecchione et al. 2020; von Ehrenstein et al. 2019, 2020; Walton and Monte 2015; Windham et al. 2006; Hamad et al. 2019; Hollowood-Jones et al. 2020; Zhu et al. 2019) as well three in Sweden (Arora et al. 2017; Gong et al. 2017; Sujan et al. 2017), two studies in Canada (Bernardo et al. 2019; Oulhote et al. 2020), two in China (Gao, Xi, Wu, et al. 2015; Gao et al. 2016), two in Denmark (Liew et al. 2015; Singer et al. 2017), two in the Netherlands (Steenweg-de Graaff et al. 2016; van den Dries et al. 2019), two in Norway (Skogheim et al. 2021; Hornig et al. 2017), two in Finland (Malm et al. 2016; Kong et al. 2020), one in Taiwan (Chen et al. 2020) and one in Israel (Rotem et al. 2020). Of the 47 studies, 34 (72.3%) used validated tools for diagnosis of ASD including the Social Responsiveness Scale (SRS) (Maenner et al. 2023; Braun et al. 2014; Goodrich et al. 2017; McGuinn et al. 2020; Hollowood-Jones et al. 2020; Zhu et al. 2019; Oulhote et al. 2020; Gao, Xi, Wu, et al. 2015), Autism Diagnostic Observation Schedule (ADOS) (Jin et al. 2020; Traglia et al. 2017; Granillo et al. 2019; Lyall et al. 2017; Millenson et al. 2017; Shin et al. 2018; Geier, Kern, and Geier 2009; Kalkbrenner et al. 2015; McCanlies et al. 2019; von Ehrenstein et al. 2020; Walton and Monte 2015), Autism Diagnosis Interview-Revised (ADI-R) (Traglia et al. 2017; Granillo et al. 2019; Millenson et al. 2017; Shin et al. 2018; Geier, Kern, and Geier 2009; Jo et al. 2019; Kalkbrenner et al. 2015; McCanlies et al. 2019; von Ehrenstein et al. 2019; Walton and Monte 2015), Childhood Autism Rating Scale (CARS), and the Diagnostic and Statistical Manual criteria for ASD diagnosis (DSM-IV/V) (Jin et al. 2020; Simkova, Veleminsky, and Sram 2020; Gómez-Roig et al. 2021; Nye et al. 2014; J. H. Kim et al. 2021; Ritz et al. 2020; Patti, Li, et al. 2021; Raz et al. 2015; Singer et al. 2016; von Ehrenstein et al. 2020; Walton and Monte 2015; Windham et al. 2006; Gong et al. 2017; Liew et al. 2015; Skogheim et al. 2021; Volk et al. 2014). The tool for diagnosis of ASD in the remaining 13 studies was either unclear or not specified.
Detailed study characteristics and results are summarized in Table 1, organized by exposure categories and alphabetically by study author. This table also details the gestational age at exposure/measurement and analyses method of each study.
Table 1.Studies examining association of pregnancy exposures with autism spectrum disorder (ASD)
| Author, Year, Location, Study Design |
Gestational
Age at
Exposure |
Population |
Metabolites/Exposures; Sample; Analysis Method |
Outcome |
Results |
| Biomarkers |
| Bernardo, 2019, Canada, cohort study |
T11
|
546 children age 3-4 years from mother-infant pairs in Maternal-Infant Research on Environmental Chemicals (MIREC) study. 8 cases with autistic behaviors |
24 polychlorinated biphenyl (PCB) congeners; maternal plasma; GC/MS2
|
Autistic behavior by SRS3-2 mean scores |
No significant associations between plasma PCB concentrations and autistic behavior
Mean SRS point increase with high levels of PCB138 was 1.4 points (95% PCI4: -0.4, 3.2) |
| Braun, 2014, US, cohort study |
16 weeks, 26 weeks, within 24 hours of delivery |
175 children age 4-5 years from pregnant women in Health Outcomes and Measures of the Environment (HOME) prospective birth cohort study. 22 cases with autistic behavior |
Endocrine-Disrupting chemicals (EDCs); maternal serum (n=60) and urine (n=10); MS
EDCs: 8 phthalate metabolites, bisphenol A, 25 PCBs, 6 organochlorine pesticides, 8 brominated flame retardants, 4 perfluoroalkyl substances |
Autistic behavior by SRS scores completed by the participants’ mothers when children were 4 and 5 years of age |
Significant increased risk association between 2 standard deviation (SD) increase in serum concentration of trans-nonachlor (β4 4.1, 95% CI: 0.8, 7.3) and autistic behaviors
Significant decreased risk associations between children born to women with detectable vs. nondetectable concentrations of β-hexachlorocyclohexane (β -3.3, 95% CI: -6.1, -0.5) or PBDE-85 (β -3.2, 95% CI: -5.9, -0.5) and autistic behavior |
| Granillo, 2019, US, cohort study |
T1, T2, T3 |
104 children age 3 years from Markers of Autism Risk in Babies-Learning Early Signs [MARBLES] cohort of families that have an older child sibling with ASD. 21 cases with ASD, 23 cases with non-TD5, 60 controls with TD6
|
PCBs; maternal plasma; GC/MS |
ASD7, non-TD, and TD by clinical diagnosis, ADOS8 and MSEL9 instruments, and DSM-410 criteria |
No significant association between maternal total PCBs (OR 1.63, 95% CI: 0.60, 4.44) and ASD |
| Liew, 2015, Denmark, nested cohort study |
T1, T2 |
990 children born during 1996 – 2003 from mother-child pairs in Danish National Birth Cohort. 220 cases with autism, 550 controls |
16 perfluoroalkyl substances (PFASs); maternal plasma; LC-MS |
Autism by previously diagnosed ICD-1011 codes |
No significant associations between mother’s PFAS plasma levels PFOS (RR 0.92, 95% CI: 0.69, 1.22) or PFOA (RR 0.98, 95% CI: 0.73, 1.31) and autism |
| Lyall, 2017, US, case-control study |
T2 (15-19 weeks) |
1,144 children born during 2000 – 2003 from mother-baby pairs in Early Markers for Autism (EMA) study. 545 cases with ASD, 181 cases with Intellectual Disabilities [ID] without ASD, 418 controls |
11 PCB congeners and 2 organochlorine (OC) pesticides; maternal serum and blood cell pellets; GC-isotope dilution high-resolution MS
PCBs: 28, 99, 118, 138/158, 153, 170, 180, 187, 194, 196/203, 199
OC pesticides: trans-nonachlor and p,p’-DDE |
ASD and ID by DSM-4-TR criteria via clinical review of electronic medical record (EMR). ASD cases qualified by presence or absence of co-occurring intellectual disability |
No significant associations between PCB 138/158 (aOR 1.54, 95% CI: 0.87, 2.71) or PCB 153 (aOR 1.72, 95% CI: 0.97, 3.07) and ASD when adjusted for genetic ancestry
Significant increased risk associations between the highest vs. lowest quartile of PCB138/158 (aOR 1.79, 95% CI: 1.10, 2.71), PCB153 (aOR 1.82, 95% CI: 1.10, 3.02), and ASD
No significant associations between OC pesticides (aOR 1.35, 95% CI: 0.93, 1.96) and ASD |
| Lyall, 2018, US, case-control study |
T2 (15-19 weeks) |
1,175 children born during 2000 – 2003 from mother-baby pairs in EMA study. 553 cases with ASD, 189 cases with ID without ASD, 433 general population controls |
8 PFASs; maternal serum; MS
PFASs: (FOSA/PFOSA, Et-FOSSA/ET-PFOSA-AcOH, Me-FOSAA/Me-PFOSA-AcOH, PFHxS, PFOS, PFOA, PFNA, PFDA/PFDeA)12
|
ASD and ID by DSM-4-TR criteria, identified from California Department of Developmental Services and confirmed by a developmental pediatrician |
Decreased risk associations between PFOA (aOR 0.62, 95% CI: 0.41, 0.93), PFOS (aOR 0.64, 95% CI: 0.43, 0.97) and ASD
Mean concentrations of most PFAS were lower in ASD and ID groups relative to controls |
| McKean, 2015, US, case-control study |
T1, T2, T3 |
257 children age 2-5 years from mother-child pairs in Childhood Autism Risks from Genetics and the Environment (CHARGE) study. 164 cases with ASD, 35 cases with Developmental Delays (DD)/non-TD, 58 controls with TD |
Mercury concentrations; newborn bloodspot sample; MS
Maternal fish consumption; questionnaire
MeHg concentration estimates determined via toxicokinetic model |
ASD by ADI-R13, ADOS-G and screened with SCQ14.
DD and TD by MSEL, Vineland Adaptive Behavior scales, and SCQ |
No significant associations between cumulative MeHg over entire gestational period, T2, or T3 (OR 0.95, 95% CI: 0.95, 1.12) and ASD |
| Millenson, 2017, US, cohort study |
T2 (16 and 26 weeks) |
224 children age 7.4-10 years from pregnant women in HOME study. 44 cases with SRS scores >60, 6 cases with SRS scores > 75 |
6 organophosphate (OP) insecticide metabolites; maternal urine samples; GC/MS
PON genotype; cord blood; TaqMan assays
OPs: DMP, DMTP, DMDTP, DEP, DETP, DEDTP12
|
Autism/ASD by SRS transformed into a T-score |
No significant association between maternal urinary OP concentrations (β 1.2, 95% CI: -4.0, 1.6)r and children’s SRS scores
No significant association between OP concentrations and SRS scores when modified by PON1 genotypes |
| Philippat, 2018, US, cohort study |
T1, T2, T3 |
203 children born during 2003 – 2014 from mother-child pairs in the MARBLES study. 46 cases with ASD, 55 cases with non-TD, 102 controls with TD |
7 OP metabolites; maternal urine samples; GC and LC/MS
OPs: DMP, DEP, DMTP, DMDTP, DETP, DEDTP, TCPy12
|
ASD, non-TD, and TD by ADOS, MSEL, SCQ, and ADI-R evaluation at 3 years |
No significant associations between maternal OP metabolites [all p-values ≥0.15] and ASD or non-TD |
| Ritz, 2020, US, case-control study |
T2 [~16 weeks] |
114 children born during 2004-2010. 52 cases with ASD, 62 controls without ASD |
Metabolites; maternal serum samples; LC-MS
Metabolites: quinoline, ornithine, 1-methylhistidine, methyl jasmonate, benzoate, nonanoic acid, and 10-hydroxydecanoate |
ASD by DSM-4
criteria using ICD-9-CM codes |
Decreased risk association between quinoline and ASD
Increased risk associations between ornithine, 1-methylhistidine, methyl jasmonate, benzoate, nonanioic acid, 10-hydroxydecanoate and ASD
Significant increased risk associations with 7 enriched metabolic pathways with all p <0.05 including glycosphingolipid biosynthesis, phosphatidylinositol phosphate metabolism, bile acid biosynthesis, N-Glycan biosynthesis, glycosphingolipid metabolism, pyrimidine metabolism, C21-steroid hormone biosynthesis and metabolism, and ASD |
| Shin, 2018, US, cohort study |
T1, T2, T3 |
201 children born during 2007 – 2014 from mother-child pairs in MARBLES study. 46 cases with ASD, 55 cases with non-TD, 100 controls with TD |
14 metabolites of eight phthalates; maternal urine samples; LC/MS
Phthalate metabolites: MEP, MiBP, MHiBP, MBP, MHBP, MBzP, MEHP, MEHHP, MEOHP, MECPP, MCPP, MNP, MCOP, MCNP12
Folic acid levels; prenatal questionnaire |
ASD by ADOS and MSEL at 3 years of age |
Significant decreased risk association between MCPP in T2 [RRR 0.53, 95% CI: 0.32, 0.87] and ASD
Significant decreased risk associations in mothers who took prenatal vitamins between MiBP [RRR 0.44, 95% CI: 0.21, 0.88], MCPP [RRR 0.41, 95% CI: 0.20, 0.83], MCOP [RRR 0.49, 95% CI: 0.27, 0.88], and ASD. However, this association was not found in mothers who did not take prenatal vitamins |
| Steenweg-de Graaf, 2016, Netherlands, cohort study |
T1, T2 |
4,624 children born during 2001 – 2006 from mother-child pairs in Generation R cohort study with available child autistic trait data |
Polyunsaturated fatty acid; maternal plasma samples; high-throughput method
Maternal fish consumption over 3 months; food frequency questionnaire |
Autistic traits by SRS and CBCL15 administered at mean child age of 6.2 years |
Significant risk association between lower maternal ω-3: ω-6 ratio [β -0.009, 95% CI: -0.017, -0.001] and child autistic traits
Significant risk associations between higher total ω-6 levels [βtotal ω-6 0.011, 95% CI: 0.002, 0.020], ω-6 linoleic acid levels [βω-6 linoleic acid 0.012, 95% CI: 0.001, 0.023] and child autistic traits
No significant association between maternal fish intake [β -0.003, 95% CI: -0.011, 0.005] and child autistic traits |
| Traglia, 2017, US, case-control study |
T2 [15 -20 weeks] |
735 children born during 2000 – 2003 from mother-infant pairs in EMA study. 366 cases with ASD, 369 controls |
21 organohalogens; maternal serum samples; GC isotope dilution in MS
Maternal and fetal ancestry DNA markers used for adjusted analyses |
ASD by study clinician expert review of records |
Significant increased risk associations between three polybrominated compounds [PBDE-100, 153, and SUM PBDE] in the ancestry-adjusted analysis, [P < 0.05] and ASD |
| Van den Dries, 2019, Netherlands, cohort study |
T1, T2, T3 |
622 children born during 2002 – 2006 from mother-child pairs with exposure and data on autistic traits at 6 years. 12 cases with autistic traits |
OP pesticides, specifically dialkyl phosphates [DAPs]; maternal urine samples; GC/MS
DAPs: DMP, DMTP, DMDTP, DEP, DETP, DEDTP |
Autistic traits by SRS administered at 6 years of age |
No significant association between higher maternal urinary concentrations of DAP metabolites [adjusted B 0.17, 95% CI: -0.20, 0.54] and autistic traits |
| Environmental exposures |
| Arora, 2017, Sweden, cohort study |
T2 through early childhood |
32 twin-pairs from The Roots of Autism and ADHD Twin Study in Sweden [RATSS]. 12 children from ASD concordant pairs, 20 children from ASD discordant pairs, 44 control children non-ASD pairs |
Biomarkers of metal exposure; children’s naturally shed deciduous teeth-matrix; laser ablation-coupled plasma mass spectrometry
Metals: manganese, zinc, lead, tin, strontium, chromium |
ASD by DSM-5 criteria corroborated by ADI-R and ADOS-2. Autistic traits by SRS-2 |
Significant increased risk associations between lower manganese levels 15 weeks postnatally [2.5 times lower, Holm-Bonferroni [HB] 95% CI: 1.1- 4.5 times lower], lower zinc levels 8 weeks prenatally [28% lower, HB 95% CI: 22-65% lower], higher lead levels 15 weeks postnatally [1.5 times higher, HB 95% CI: 0.9, 2.5], higher Tin levels 20-16 weeks prenatally, higher Strontium levels 22-30 weeks postnatally, and lower Chromium levels 20-15 weeks prenatally and ASD. |
| Gao, 2015, China, case-control study |
Pre-conception through birth |
926 children age 3-18 years from 11 special education schools. 193 cases with autism, 733 controls with TD |
Maternal exposures during pregnancy [air condition, microwave, computer, X-ray, smoking, stress, depression, folic acid], and delivery complications [infant gestational age, wrap of umbilical cord around neck, cesarean delivery, breech birth, newborn complications]; questionnaire |
Autism by clinical diagnosis, CARS16 score ≥30 and DSM-4 criteria |
Significant decreased risk associations between maternal use of air conditioner during pregnancy [OR 0.316, 95% CI: 0.22, 0.46 and ASD
Significant increased risk associations between maternal depression [OR 4.822, 95% CI: 3.08, 7.63], newborn complications at delivery [OR 4.277, 95% CI: 2.31, 7.91], and ASD |
| Gao, 2016, China, case-control study |
6 months pre-conception through birth |
295 children age 4-17 years. 108 cases with ASD, 79 cases with ID, 108 matched controls |
Parental fish consumption [and other products like meat, eggs, fruits, vegetables, and alcohol]; questionnaire |
ASD by DSM-4 criteria. ID assessed with Wechsler Young Children Scale of Intelligence. ASD and ID identified from the registry of special education schools |
Significant decreased risk associations between maternal preference for eating grass carp fish [Exp[B] 0.279, 95% CI: 0.095, 0.799] or fruits [Exp[B] 0.413, 95% CI: 0.216, 0.804] during pregnancy, and ASD |
| Geier, 2009, US, cohort study |
Pregnancy |
100 children age >6 years. 60 cases with ASD, 40 cases with autism |
Maternal dental amalgams; medical history |
ASD or autism by ATEC17, ICD-9
ASD designating mild condition, autism designating severe condition |
No significant association between mean number of amalgams and patients with ASD [mild] vs. autism [severe] [χ2=2.8, df=1, p=0.0946]
Children of mothers w/ >6 amalgams have 3.2x greater odds of autism vs. ASD diagnoses than those with <5 amalgams [χ2=6.2, df=1, p=0.0127] |
| Gong, 2017, Sweden, case-control study |
Pregnancy and child’s first year |
23,373 children born during 1993 – 2007. 5,136 cases with ASD, 18,237 controls |
Air pollution from road traffic [NOx and PM10]; residential addresses; Gaussian air quality dispersion model estimating temporal and spatial distribution with an R2 of 0.74-0.80 |
ASD by ICD-9/10 codes and DSM-4 codes, identified through health registers |
No significant associations between air pollution during prenatal period per 10 µg/m3 increase in PM10 [aOR 1.00, 95% CI: 0.86, 1.15] or 20 µg/m3 increase in NOx [aOR 1.02, 95% CI: 0.94, 1.1] and ASD
Significant inverse association between air pollution exposure among children whose mothers moved to a new residence during pregnancy [p < 0.03] and ASD |
| Goodrich, 2018, US, case-control study |
3 months pre-conception through breastfeeding |
606 children born during 1998 – 2007 from mother-child pairs in CHARGE case-control study. 346 cases with ASD, 260 controls with TD |
Average total daily folic acid [FA] intake each month; parental interview
Regional exposure to near roadway air pollution [NRP], NO2, Ozone [O3], PM2.5, and PM10; residential addresses; California LINE4 air space dispersion model |
ASD by ADOS scores above the cutoff and meeting criteria on ADI-R
TD by SCQ screen |
Significant difference between periconceptual FA intake levels with high NO2 exposure during T1 [NO2 and low FA intake OR 1.53, 95% CI: 0.91, 2.56 vs. NO2 and high FA intake OR 0.74, 95% CI: 0.46, 1.19, P-interaction = 0.04] and ASD
No significant associations between other pollutant exposures and ASD after adjustment |
| Jo, 2019, US, cohort study |
Preconception through 1 year postnatal |
246,420 children age 1-5 years. 2,471 cases with ASD, 243,949 controls |
Air pollutants O3, PM2.5, PM10, and NO2 ; Birth certificate residential addresses; EPA air quality monitoring network
Diabetes; ICD-9 codes, anti-diabetic medication use, and glucose values from 50-g, 100-g or 75-g oral glucose challenge tests |
ASD by ICD-9 codes or equivalent codes from EMR in at least 2 visits |
No significant associations between O3, PM10, or NO2 exposure and ASD
Significant increased risk association between PM2.5 exposure during preconception, T1, T3, entire pregnancy [HR 1.17, 95% CI: 1.04, 1.33], or the first year of life and ASD
Significant increased risk association between children born to mothers with pre-existing DM2 [HR 1.60, 95% CI: 1.26, 2.16] and ASD |
| Kalkbrenner, 2015, US, case-control study |
3 months pre-conception through 1 year postnatal |
15,647 children born during 1994 – 2000. 979 cases with autism, 14,668 controls |
Traffic-related air pollution PM10; Birth addresses; geostatistical interpolation method |
Autism by DSM-4-TR criteria. Identified by records-based surveillance programs and reviewed by health and educational agencies |
Significant decreased risk association between 10 µg/m3 increase in PM10 exposure in T1 [aOR 0.86, 95% CI: 0.74, 0.99] and autism
Significant increased risk association between 10 µg/m3 increase in PM10 exposure in T3 [aOR 1.36, 95% CI: 1.13, 1.63] and autism |
| Kim, 2021, US, case-control study |
T1 through mid-pregnancy |
214 children born during 2005-2010 to mothers with high levels of air pollution exposure during pregnancy. 116 cases with ASD, 98 controls without ASD |
389 metabolites; maternal serum; liquid chromatography [LC]-MS
Traffic air pollutant exposure to CO, Nitrogen oxides [NOx] and particulate matter with diameter <2.5µm [PM2.5]; geocoded residential addresses on birth certificates |
ASD by DSM-4 criteria using ICD-9-CM codes |
Hypotaurine and urate positively associated, while phenylalanine and 3-hydroxybutanoic acid were negatively associated with ASD in infants |
| McCanlies, 2019, US, case-control study |
3 months pre-conception through birth |
951 children age 2-5 years from mother-child pairs in CHARGE study in 2003. 537 cases with ASD, 414 controls with TD |
Industrial hygienists estimated occupational exposure to 16 agents; parental interview assessing workplace exposure based on frequency [0-3] and intensity [0-3] of exposure |
ASD by ADOS and ADI-R. All children evaluated via MSEL, VABS18
TD by SCQ, MSEL and VABS |
Significant increased risk association between occupational solvent exposure [OR 1.5, 95% CI: 1.01, 2.23] and ASD
No significant risk associations between other exposures and ASD |
| McGuinn, 2020, US, case-control study |
3 months pre-conception through 1 year postnatal |
1,529 children born during 2003-2006 from mother-child pairs in the Study to Explore Early Development [SEED]. 674 cases with ASD, 855 controls |
Regional exposure to PM2.5 and Ozone [O3]; residential addresses at birth; Goddard Earth Observing System of the NASA Global Modeling and Assimilation Office exposure prediction model |
ASD by screening with SCQ and diagnosis with ADOS and ADI-R |
Significant increased risk association between O3 exposure during T3 [OR 1.2, 95% CI: 1.1, 1.4] and ASD per interquartile range width of 6.6 parts per billion
Significant increased risk association between PM2.5 exposure during T3 [OR 1.3, 95% CI: 1.0, 1.6] and ASD per 1.6 µg/m3 increase in PM2.5
|
| Oulhote, 2020, Canada, cohort study |
T1 |
510 children age 3-4 years from MIREC study. 2 cases with ASD, 11 cases with deficiencies in social behavior |
Phthalate metabolites; Maternal urine; LC-MS/MS
Phthalate metabolites: MEP, MBP, MBzP, MCPP, MCHP, MOP, MNP, MMP, DEHP, MEHP, MEHHP, MEOHP
Prenatal folic acid [FA]; questionnaire |
ASD and deficiencies in social behavior by SRS-2 |
Significant increased risk associations between a 2-fold increase in urinary MBP and MCPP concentrations and total SRS score increases of 0.6 points [95% CI: 0.1, 1.0] and 0.5 points [95% CI: 0.1, 0.8] respectively
Significant decreased risk association in female children between two-fold increase in MEP concentrations and SRS score decreases of -0.4 points [95% CI: -0.7, 0.0]
Significant attenuation of associations between phthalate concentrations and higher SRS scores for DEHP, MCPP, and MBP when FA supplemented > 400µg/day [p<0.1] |
| Patti, 2021, US, cohort study |
T1 through T3 |
389 children age 3-8 years; 120 mother-child pairs from mothers in Early Autism Risk Longitudinal Investigation [EARLI] prospective birth cohort study with 20 cases of ASD. 269 pairs from the HOME study with 43 cases of ASD |
Caffeine intake; maternal questionnaire; caffeine quantities determined by USDA nutrient databases and product websites
Sources of caffeine: coffee, tea, soda, chocolate |
ASD by SRS |
No significant risk associations between caffeine intake in the EARLI cohort [β per IQR increase [57mg]: 2.0, 95% CI: -0.1, 4.0], HOME cohort [β per IQR increase [0.43mg]: 0.6, 95% CI: -0.5, 1.6] and SRS T-scores
Significant increased risk association between average caffeine intake and SRS scores in the pooled sample data [β per IQR increase: 1.2, 95% CI: 0.3, 2.1] |
| Raz, 2015, US, case-control study |
Not specified |
1,146 children born during 1990 – 2002 from mothers in Nurses’ Health Study II. 160 cases with ASD, 986 randomly selected controls without ASD |
PM2.5 and PM10-2.5 air pollution; birth addresses; spatiotemporal model |
ASD by maternal report and validated against ADI-R |
Significant risk association between PM2.5 exposure per interquartile range [IQR] increase in PM2.5 during pregnancy [aOR 1.57, 95% CI: 1.09, 1.86] and ASD, among women with the same address before and after pregnancy. Most significant during T3
No significant associations between PM2.5 during T1 or T2, or between PM10-2.5 and ASD |
| Schmidt, 2017, US, case-control study |
3 months pre-conception through birth |
806 children born during 2000 – 2007 from mother-child pairs in CHARGE case-control study. 466 cases with ASD, 340 controls without ASD |
Maternal FA and vitamin intake, and indoor household pesticide exposure; telephone interview [High FA intake defined as ≥800 µg during the first pregnancy month]
Commercial agricultural pesticide exposure [carbamate, OC, OP, and pyrethroid]; geocoded gestational addresses by statewide database; ArcGIS special model |
ASD by ADOS, parents given ADI-R
Controls by SCQ |
Significant increased risk association between the combination of low FA intake and indoor pesticide use [aOR 2.5, 95% CI: 1.3, 4.7], high FA intake and indoor pesticide use [aOR 1.7, 95% CI: 1.1, 2.8] and ASD
No significant association between any agricultural pesticide exposure in high FA or low FA group [low FA OR 2.0, 95% CI: 0.9, 4.2] and ASD |
| Singer, 2016, US, case-control study |
Any |
1,866 Children age 30-68 months from SEED cohort whose mothers reported ≥1 job during pregnancy or breastfeeding. 463 cases with ASD, 693 children with DDs, 710 controls from the general population |
Occupational exposure to asthma-causing agents [asthmagens]; maternal interview; job-exposure matrix [JEM]
Asthmagen sub-groups: high molecular weight agents [HMW], low molecular weight agents [LMW], mixed environments, irritants |
ASD screened by SCQ and confirmed by ADI-R and ADOS |
No significant association between occupational asthmagen exposure [aOR 1.39, 95% CI: 0.96, 2.02] and ASD
13.9% of mothers were exposed to asthmagens during pregnancy, most commonly latex antigens, highly reactive agents, and cleaning/disinfectant products |
| Singer, 2017, Denmark, case-control study |
Not specified |
Children born during 1993 – 2007 from Danish Civil Registration System who are the oldest child in their family. 11,869 cases with ASD, 48,046 controls |
Occupational exposure to asthmagens; Denmark occupational register; linking Danish International Standard Classification of Occupations [DISCO-88] codes to an asthma-specific job-exposure matrix [JEM] |
ASD by ICD-10 codes recorded in Danish Psychiatric Central Register [DPCR] |
Significant decreased risk association between maternal asthmagen exposure [aOR 0.92, 95% CI: 0.86, 0.99] and ASD
Significant decreased risk association between latex exposure [aOR 0.90, 95% CI: 0.82, 0.98] and ASD
19.8% of mothers were exposed to occupational asthmagens |
| Skogheim, 2021, Norway, cohort study |
Mid-pregnancy |
1,431 children born during 2002-2009 from child-mother pairs in Norwegian Mother, Father and Child Cohort Study [MoBa]. 397 cases with ASD, 1034 controls |
Metals and essential elements; maternal serum; MS
Metals/elements: arsenic, cadmium, cesium, cobalt, copper, lead, mercury, magnesium, manganese, selenium, zinc |
ASD by ICD-10 codes in the Norwegian Patient Registry [NPR] of “pervasive developmental disorders” |
Significant increased risk associations between second quartile of arsenic [OR 1.77, 95% CI: 1.26, 2.49], and the fourth quartiles of cadmium [OR 1.57, 95% CI: 1.07, 2.31] and manganese [OR 1.84, 95% CI: 1.30, 2.59] and ASD
Significant decreased risk associations between second quartile of copper [OR 0.69, 95% CI: 0.49, 0.98], fourth quartiles of cesium [OR 0.63, 95% CI: 0.44, 0.91] and zinc [OR 0.63, 95% CI: 0.45, 0.90], and second/third/fourth quartiles of mercury [OR 0.43-0.56, 95% CI 0.30, 0.80] and ASD |
| Vecchione, 2021, US, cohort study |
1-20 weeks, 21-36 weeks |
156 children age <3 years from mothers in EARLI cohort. 270 children age <12 years from HOME cohort |
EARLI: Fish intake; maternal food frequency questionnaires [FFQs] modified from the National Cancer Institute’s Dietary History Questionnaire [DHQ]
HOME: Fish intake; maternal questionnaire |
ASD-related traits by SRS
Cognitive at 36 months by MSEL ELC 1 [in EARLI cohort] and Bayley Scales of Infant Development MDI19, Second edition [in HOME cohort] |
Significant increased risk association between higher fish intake during 21-36 weeks of pregnancy [β 5.60, 95% CI: 1.03, 17.86] and increased SRS scores
No significant risk association between high fish intake [as compared to no intake] and higher MSEL ELC scores in EARLI [β 6.55, 95% CI: -1.94, 15.04] or HOME MDI scores [β -0.78, 95% CI: -5.86, 4.31] |
| Von Ehrenstein, 2019, US, case-control study |
3 months pre-conception through 1 year postnatal |
38,331 children born during 1998 – 2010 from mother-child pairs in California Department of Developmental Services and the Office of Vital Statistics. 2,961 cases with ASD, 35,370 controls |
11 agricultural pesticides; geocoded residential birth addresses; CA-PUR reports and California Department of Water Resources |
ASD by DSM-4-R, recorded in Department of Developmental Services registry [code 299.00] |
Significant increased risk associations between chlorpyrifos [OR 1.15, 95% CI: 1.20, 1.29], diazinon [OR 1.14, 95% CI: 1.03, 1.26], avermectin [OR 1.14, 95% CI 1.03, 1.26], and ASD |
| von Ehrenstein, 2020, US, case-control study |
3 months pre-conception through T3 |
2,015,104 children born during 2007 – 2010 from mother-child pairs reported in California Office of Vital Statistics and Department of Developmental Services. 11,722 cases with ASD and 2,003,382 controls without ASD |
Maternal smoking; California birth records
[Heavy smoking defined as ≥20 cigarettes/day] |
ASD by DSM-4-TR criteria |
Significant increased risk association between maternal smoking [OR 1.15, 95% CI: 1.04, 1.26] and ASD
Significant increased risk association with heavy maternal smoking during pregnancy [OR 1.55, 95% CI: 1.21, 1.98] and ASD. Similar associations found in T1, T2, or T3 individually |
| Walton, US, 2015, case-control study |
Not specified |
711 children born after 1984. 161 cases with autism, 550 controls without autism |
Average weekly methanol consumption; maternal questionnaire |
Autism by maternal reporting |
Significant increased risk association between reported maternal methanol consumption [p= 5.0013e-26] and autism |
| Windham, 2006, US, case-control study |
Not specified |
941 children born in 1994. 284 cases with ASD, 657 controls |
19 hazardous air pollutant [HAP] concentrations; birth locations via U.S. census tract level; Gaussian air dispersion model
Groups of HAPs: chlorinated solvents, heavy metals, aromatic solvents |
ASD by DSM-4 criteria before children’s ninth birthday |
Significant increased risk associations between heavy metals in the third quartile [aOR 1.95, 95% CI: 1.2-3.1] and fourth quartile [aOR 1.7, 95% CI: 1.0-3.0] and ASD |
| Windham, 2013, US, case-control study |
Not specified |
943 children born in 1994. 284 cases with ASD from autism surveillance program, 659 population controls |
Occupation exposure to 8 exposure/chemical groups; birth certificates self-reported occupations
Exposures: exhaust/combustion products, solvents, pesticides, metals, disinfectants, auto paint, cooling fluid, and summary variable exposure |
ASD by DSM-4 criteria by expert clinician review |
Significant increased risk association between mothers working in occupations considered exposed [aOR 2.3, 95% CI: 1.3, 4.2] and ASD
Most significant increased risk associations between exhaust and combustion products [aOR 12.0, 95% CI: 1.4, 104.6], disinfectants [aOR 4.0, 95% CI: 1.4, 12.0] and ASD.
14.4% of mothers of cases with ASD were exposed to occupational exposures vs. 7.2% of controls |
| Medication exposures |
| Hamad, 2019, US, cohort study |
T1, T2, T3 |
214,834 children born during 1998 – 2016 from Manitoba Population Research Data Repository. 2,965 cases with ASD |
Antibiotic prescription dispensation; Drug Program Information Network information |
ASD by ICD-9 or ICD-10 codes, or the presence of an “ASD” identifier in the Manitoba Education and Training Special Needs Funding data |
Significant increased risk association between antibiotic exposure [aHR 1.10, 95% CI: 1.01, 1.19] and ASD
37.6% of children were exposed to antibiotics prenatally |
| Malm, 2016, Finland, cohort study |
Any |
64,754 children born during 1996 – 2010 to women in Finland National Register. 307 cases with ASD |
Selective Serotonin Reuptake Inhibitor [SSRI]; Hospital Discharge Register and Drug Reimbursement Register
Exposure categories: SSRI exposed; exposed to psychiatric disorder, no antidepressant; SSRI exposed only before pregnancy; unexposed to antidepressants and psychiatric disorders |
ASD by ICD codes from birth to 14 years |
Significant increased risk association between SSRI exposure [aHR 1.40, 95% CI: 1.02, 1.92] and ASD [compared to unexposed to antidepressants and psychiatric disorder group]
Significant increased risk association between exposed to psychiatric disorder, no antidepressant [aHR 1.59, 95% CI: 1.16, 2.18] and ASD [compared to unexposed to antidepressant and psychiatric disorder group] |
| Sujan, 2017, Sweden, case-control study |
T1 |
1,580,629 children born during 1996 – 2012 from population-based Swedish registries. 14,617 cases with ASD |
Antidepressant; Maternal self-reported history and the Prescriber Drug Register records |
ASD by ICD-9 and ICD-10 criteria |
No significant association between T1 antidepressant exposure [aHR 0.83, 95% CI: 0.62, 1.13] and ASD
1.4% maternal T1 self-reported antidepressant use |
| Genetic variability |
| Hollowood-Jones, 2020, US, case- control study |
Not specified |
59 children age 2-5 from mother-child pairs in Arizona State University’s Autism/Asperger’s Research Program and Zoowalk for Autism Research. 30 cases with ASD, 29 TD controls |
Vitamin B12, folate, methylmalonic acid, homocysteine, isoprostane, vitamin D, vitamin E, hCG, methylenetetrahydrofolate reductase gene variants, ferritin, and thiols; maternal urine and blood; various assays, LC-MS/MS, and LC |
ASD by ADI-R |
No significant risk associated with MTHFR mutation A1298C [p=0.15] or C677T [p=0.90] and ASD
Significant increased risk associations between lower vitamin B12 level [Ratio 0.75, p<0.00], SAM/SAH20 ratios [Ratio 0.93, p=0.01], 4-vinylphenol sulfate [Ratio 0.31, p<0.00], NAD+ [Ratio 0.41, p=0.03], gamma-glutamylglycine [Ratio 0.27, p<0.00], cinnamoylglycine [Ratio 0.46, p=0.01], propionylglycine [Ratio 0.48, p=0.01], carnitine-conjugated metabolites [Ratios 0.63-0.87, p ≤0.03] and ASD
Significant increased risk associations between higher levels of Glu-Cys [Ratio 1.10, p=0.01], fCysteine [Ratio 1.06, p=0.01], fCystine [Ratio 1.07, p=0.02], dimethyl sulfone and ASD [Ratio 18.7, p=0.01]
and ASD |
| Zhu, 2019, US, cohort study |
Not specified |
41 male children from MARBLES cohort. 20 cases with ASD, 21 controls with TD |
Differently methylated regions [DMRs] of the placenta; placenta from birth; whole methylome analyses of placenta |
ASD by ADOS cutoff and DSM-5
TD by MSEL and ADOS |
400 potential DMRs with >10% methylation difference between ASD and TD groups
ASD DMRs enriched around placental H3K4me3 [OR 17.08, FDR q <1.8E-42] and brain H3K4me3 [OR 13.75, FDR q<3.55E-31] marks that regulate gene function, and TSS flanking regions
2 placental ASD DMRs at CYP2E1 [chr10] and IRS2 [chr13]. IRS2 methylation is sensitive to maternal prenatal vitamin intake |
| Maternal illness |
| Chen, 2020, Taiwan, cohort study |
Pre-conception through 3 years postnatal |
708,515 children born during 2001-2008 from Taiwan National Health Insurance Research Database. 4506 cases with ASD |
Depressive disorders; ICD-9 diagnostic codes |
ASD by ICD-9 codes |
Significant increased risk associations between maternal depression before pregnancy [HR 2.01, 95% CI: 1.70, 2.37], during pregnancy [HR 1.58, 95% CI: 1.11, 2.25], postnatal [HR 1.59-1.65, 95% CI: 1.29, 1.81] and ASD |
| Hornig, 2018, Norway, cohort study |
13 weeks through 6 months postnatal |
95,754 children born during 1999 – 2009 from MoBa cohort. 583 cases with ASD, 95,171 controls without ASD |
Maternal fever [>38.5 C] and antipyretic use; maternal questionnaire |
ASD by DSM-4-TR criteria or ICD-10 [84 code] |
Significant increased risk associations between maternal fever any time during pregnancy [aOR 1.34, 95% CI: 1.07, 1.67] or during specifically T2 [aOR 1.40, 95% CI: 1.11, 1.77] and ASD
Significant increased risk associations between exposure to higher frequency of fevers after 12 weeks gestation [one to two fever episodes [aOR 1.30, 95% CI: 1.02, 1.66], greater than or equal to 3 fever episodes [aOR 3.12, 95% CI: 1.28, 7.63]] and ASD
Significant increased risk association between fever without acetaminophen use in T2 [OR 1.46, 95% CI 1.03, 2.05] and ASD. No significant associations in other trimesters |
| Kong, 2020, Finland, cohort study |
Not specified |
647,099 children born during 2004 – 2014 from Finland nationwide registries. 2346 cases with ASD |
BMI: Maternal BMI; first prenatal visit records via the Drugs and Pregnancy Database [Overweight defined as BMI ≥25 to <30, obese as BMI ≥30 to <35, severely obese as ≥35]
Diabetes: maternal pre-gestational diabetes and purchase of insulin records obtained from the Finnish Register on Reimbursement Drugs
[Diagnosis of type 2 diabetes [DM2] and gestational diabetes [gDM] identified from Finnish Care Registers for Health Care [HILMO] and the Finnish Register on Reimbursement Drugs based on ICD-10 codes] |
ASD by ICD-10 codes in the Finnish Care Register for Health Care |
Significant increased risk associations between insulin-treated pregestational diabetes in obese women [HR 3.61, 95% CI: 1.61, 8.07], severely obese women [HR 5.93, 95% CI: 2.81, 12.52] and ASD
Significant increased risk association between DM2 in severely obese women [HR 2.28, 95% CI: 1.18, 4.41] and ASD
Significant increased risk associations between gestational diabetes in overweight women [HR 1.28, 95% CI: 1.07, 1.53], obese women [HR 1.57, 95% CI: 1.26, 1.95] and ASD |
| Rotem, 2021, Israel, cohort study |
Not specified |
437,222 children born during 1999-2013 from mother-child pairs in the Maccabi Health Services [MHS] organization. 4,022 cases with ASD |
Polycystic Ovarian Syndrome [PCOS]; MHS database identifying ICD-9 code for maternal PCOS [256.4] and lab results for total testosterone, androstenedione, dehydroepiandrosterone sulfate, Luteinizing Hormone and Follicle Stimulating Hormone
Other androgen-related conditions; MHS database identifying ICD-9 codes for anterior pituitary hyper-function [253.1], hyperaldosteronism [255.1] Cushing syndrome [255.0], acromegaly and gigantism [253.0], other ovary hyperfunction [256.1], congenital adrenal hyperplasia [255.2], acne [706.1], hirsutism [701.1], and infertility [628.x] |
ASD by ICD-9 codes and DSM-4 and DSM-5 criteria |
Significant increased risk association between women diagnosed preconceptionally with PCOS [OR 1.42, 95% CI: 1.24, 1.64] and ASD
Significant increased risk associations between mothers with any condition of hyperandrogenemia [OR 1.41, 95% CI: 1.10, 1.79], hirsutism [OR 1.30, 95% CI: 1.10, 1.53], acne [OR 1.16, 95% CI: 1.08,1.25], infertility [OR 1.19, 95% CI: 1.10, 1.28], and ASD |
1T1: 1st trimester of pregnancy. T2: 2nd trimester of pregnancy. T3: 3rd trimester of pregnancy
2GC/MS, LC/MS: Gas chromatography/mass spectrometry, Liquid chromatography/mass spectrometry
3SRS: Social Responsiveness Scale. A score of 60-65 = ‘mild’ autistic behaviors indicative of clinically significant deficiencies in reciprocal social behavior, 66-75 = ‘moderate,’ behaviors, >75 = ‘severe’ behaviors consistent with ASD diagnosis. SRS-2 is the second edition published in 2012
4PCI and β: Posterior credible intervals for model parameters, which are the Bayesian [β] equivalent of frequentist confidence intervals [CIs]. In Bayesian confidence interval interpretation, confidence intervals containing 0 indicate non-significance
5Non-TD: Non-typically developing. Defined in Granillo et al. as not meeting criteria for ASD, but with more than one MSEL9 score 1.5 SD below the mean or at least one score 2 SD below the mean, or a high ADOS8 score
6TD: Typically developing. Defined in Gao et al. 2015 as normal intelligence, normal development, and no physical and mental illness
7ASD: autism spectrum disorder diagnosed based on developmental and behavioral history utilizing several screening and diagnostic criteria assessments
8ADOS, ADOS-G: Autism Diagnostic Observation Schedule, a semi-structured, standardized diagnostic test for ASD, published by Western Psychological Services [WPS] in 2000. In ADOS Modules 1 through 4, scores are compared with cut-off scores to yield one of three classifications: Autism, Autism Spectrum, and Non-Spectrum. Autism indicates more pronounced symptoms than Autism Spectrum. ADOS-G is a subsequent assessment with some improvements in the diagnostic accuracy
9MSEL: Mullen Scales of Early Learning, a standardized assessment that evaluates the learning abilities in various developmental areas [gross motor, fine motor, visual reception, receptive language, expressive language] in children ages 2 to 5 ½ years
10DSM-4: Diagnostic and Statistical Manual of Mental Disorders 4th edition, published in 1994. Note: DSM-4-TR is the text revision version of the DSM-4 edited in 2000. DSM-5 is the 2013 updated version of the manual
11ICD-9: International Classification of Diseases, Ninth Revision. Note: ICD-10 replaced ICD-9 in 2015
12Metabolite and exposure abbreviations: FOSA/PFOSA: perfluorooctane sulfonamide, Et-FOSSA/ET-PFOSA-AcOH: 2-[N-ethylperfluorooctane sulfonamido] acetate, Me-FOSAA/Me-PFOSA-AcOH: 2-[N-methyl-perfluorooctane sulfonamido] acetate, PFHxS: perfluorohexane sulfonate, PFOS: perfluorooctane sulfonate, PFOA: perfluorooctanoate, PFNA: perfluo-rononanoate, PFDA/PFDeA: perfluorodecanoate, DMP: Dimethylphosphate, DMTP: dimethylthiophosphate, DMDTP: dimethyldithiophosphate, DEP: diethylphosphate, DETP: diethylthiophosphate, DEDTP: diethyldithiophosphate, TCPy: 3,4,6-trichloro-2-pyridinol, MEP: monoethyl phthalate, MiBP: mono-isobutyl phthalate , MHiBP: monohydroxy-isobutyl phthalate, MBP: mono-n-butyl phthalate, MHBP: monohydroxy-n-butyl phthalate, MBzP: mono-benzyl phthalate, MEHP: monoethylhexyl phthalate, MEHHP: mono-[2-ethyl-5-hydroxyhexyl] phthalate, MEOHP: mono-[2-ethyl-5-oxohexyl] phthalate, MECPP: mono[2-ethyl-5-carboxypentyl] phthalate, MCPP: mono-3-carboxypropyl phthalate, MNP: mono-isononyl phthalate, MCOP: mono-carboxyisooctyl phthalate, MCNP: monobenzyl phthalate
13ADI-R: Autism Diagnostic Interview-Revised, a structured interview used for diagnosing autism, planning treatment, and distinguishing autism from other developmental disorders, published in 2003
14SCQ: Social Communication Questionnaire, a brief instrument evaluates communication skills and social functioning in children who may have autism or autism spectrum disorders, published in 2003
15CBCL: Child Behavior Checklist, a screening tool for ASD in clinical settings that includes a scale of pervasive developmental problems
16CARS: Childhood Autism Rating Scale, a validated autism assessment published in 2010
17ATEC: The Autism Treatment Evaluation Scale is a 77-item diagnostic assessment tool developed at the Autism Research Institute primarily to evaluate autism treatment effectiveness
18VABS: Vineland Adaptive Behavior Scales, a standardized assessment tool to measure adaptive behavior and support the diagnosis of intellectual and developmental disabilities, autism, and developmental delays
19MDI: Mental Development Index: Bayley scale designed to assess cognition through evaluation of sensory-perception, knowledge, memory, problem solving, and early language development
20SAM/SAH ratio: S-adenosylmethionine [SAM]: S-Adenosyl-L-homocysteine [SAH] is frequently used as an indicator of cellular methylation capacity
The concise summary of exposures and findings of associations with increased and decreased rates of ASD are presented in Table 2, organized again by exposure categories.
Table 2.Summary of pregnancy exposures and biomarkers associated with increased and decreased risk of ASD in the offspring
|
ASD Cases/Total Participants (Number of Combined Studies) |
Articles |
Results |
| Biomarkers |
|
|
|
| Benzoate |
52/114 (1) |
Ritz 2020 |
↑ |
| Endocrine-disrupting chemicals |
22/175 (1) |
Braun 2014 |
↑/↓ |
| Enriched metabolic pathways [7] |
52/114 (1) |
Ritz 2020 |
↑ |
| 10-hydroxydecanoate |
52/114 (1) |
Ritz 2020 |
↑ |
| Methyl jasmonate |
52/114 (1) |
Ritz 2020 |
↑ |
| 1-methylhistidine |
52/114 (1) |
Ritz 2020 |
↑ |
| Methylmercury |
164/257 (1) |
McKean 2015 |
↔︎ |
| Nonanoic acid |
52/114 (1) |
Ritz 2020 |
↑ |
| Organochlorine pesticides |
545/1,144 (1) |
Lyall 2017 |
↔︎ |
| Organohalogens |
366/735 (1) |
Traglia 2017 |
↑ |
| Organophosphates |
64/1,049 (3) |
Millenson 2017, Philippat 2018, Van de Dries 2019 |
↔︎,↔︎,↔︎ |
| Ornithine |
52/114 (1) |
Ritz 2020 |
↑ |
| Perfluoroalkyls |
773/2,165 (2) |
Liew 2015, Lyall 2018 |
↔︎,↓ |
| Phthalates |
48/711 (2) |
Oulhoute 2020, Shin 2018 |
↑/↓,↓ |
| Polychlorinated biphenyls |
574/2,444 (3) |
Bernardo 2019, Granillo 2019, Lyall 2017 |
↔︎,↔︎,↑ |
| Polyunsaturated fatty acids |
NA/4,624 (1) |
Steenweg-de Graaf 2016 |
↑ |
| Quinoline |
52/114 (1) |
Ritz 2020 |
↓ |
| Environmental exposure |
|
|
|
| Agricultural pesticides |
2,961/38,331 (1) |
Von Ehrenstein 2019 |
↑ |
| Air conditioning |
193/926 (1) |
Gao 2015 |
↓ |
| Arsenic |
397/2,136 (1) |
Skogheim 2021 |
↑ |
| Asthmagens |
12,332/61,781 (2) |
Singer 2016, Singer 2017 |
↔︎, ↓ |
| Caffeine |
63/389 (1) |
Patti 2021 |
↔︎/↑ |
| Cadmium |
397/2,136 (1) |
Skogheim 2021 |
↑ |
| Cesium |
397/2,136 (1) |
Skogheim 2021 |
↓ |
| Chromium |
10/32 (1) |
Arora 2017 |
↑ |
| Cigarette smoking |
11,722/2,015,104 (1) |
von Ehrenstein 2020 |
↑ |
| Commercial pesticides |
466/806 (1) |
Schmidt 2017 |
↔︎ |
| Copper |
397/2,136 (1) |
Skogheim 2021 |
↓ |
| Heavy metals |
284/941 (1) |
Windham 2006 |
↑ |
| High folic acid intake levels |
812/1,412 (2) |
Goodrich 2018, Schmidt 2017 |
↑,↔︎ |
| 3-hydroxybutanic acid |
116/214 (1) |
Kim 2021 |
↓ |
| Hypotaurine |
116/214 (1) |
Kim 2021 |
↑ |
| Indoor household pesticides |
466/806 (1) |
Schmidt 2017 |
↑ |
| Lead |
10/32 (1) |
Arora 2017 |
↑ |
| Manganese [low levels] |
407/2,168 (2) |
Arora 2017, Skogheim 2021 |
↑, ↑ |
| Maternal dental amalgams |
100/100 (1) |
Geier 2009 |
↔︎/↑ |
| Maternal fish consumption |
NA/5,345 (3) |
Gao 2016, Steenweg-de Gaaf 2016, Vecchione 2020 |
↓,↔︎,↑ |
| Maternal fruit consumption |
108/295 (1) |
Gao 2016 |
↓ |
| Mercury |
397/2,136 (1) |
Skogheim 2021 |
↓ |
| Methanol |
161/711 (1) |
Walton 2015 |
↑ |
| Near roadway air pollution |
346/606 (1) |
Goodrich 2018 |
↔︎ |
| Newborn complications at delivery |
193/926 (1) |
Gao 2015 |
↑ |
| Nitrogen oxides |
7,953/265,263 (3) |
Gong 2017, Goodrich 2018, Jo 2019 |
↔︎, ↑,↔︎ |
| Occupational exposures |
284/941 (1) |
Windham 2013 |
↑ |
| Ozone |
3,491/248,555 (3) |
Goodrich 2018, Jo 2019, McGuinn 2020 |
↔︎,↔︎,↑ |
| Particulate matter |
9,766/288,721 (6) |
Gong 2017, Goodrich 2018, Jo 2019, Kalkbrenner 2015, McGuinn 2020, Raz 2015 |
↔︎,↔︎,↑/↔︎,↑/↓,↑,↑ |
| Phenylalanine |
116/214 (1) |
Kim 2021 |
↓ |
| Solvents |
821/1,892 (2) |
McCanlies 2019, Windham 2006 |
↑,↑ |
| Strontium |
10/32 (1) |
Arora 2017 |
↑ |
| Tin |
10/32 (1) |
Arora 2017 |
↑ |
| Urate |
116/214 (1) |
Kim 2021 |
↑ |
| Zinc |
407/2,168 (2) |
Arora 2017, Skogheim 2021 |
↑,↓ |
| Medication exposure |
|
|
|
| Antibiotics |
2,965/214,834 (1) |
Hammad 2019 |
↑ |
| Antidepressants |
14,924/1,645,383 (2) |
Malm 2016, Sujan 2017 |
↑,↔︎ |
| Genetic variability |
|
|
|
| MTHFR mutation A1298C or C677T |
30/59 (1) |
Hollowood-Jones 2020 |
↔︎ |
| Carnitine-conjugated metabolites [lower levels] |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| Cinnamoylglycine [lower levels] |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| fCysteine |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| fCystine |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| Dimethyl sulfone |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| Gamma-glutamylglycine [lower levels] |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| Glu-Cys |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| NAD+ [lower levels] |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| Placental DMRs |
20/41 (1) |
Zhu 2019 |
↑ |
|
PON genotype |
6/224 (1) |
Millenson 2017 |
↔︎ |
| Propionylglycine [lower levels] |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| SAM/SAH [lower levels] |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| 4-vinylphenol sulfate [lower levels] |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| Vitamin B12 [lower levels] |
30/59 (1) |
Hollowood-Jones 2020 |
↑ |
| Maternal illness |
|
|
|
| Acne |
4,022/437,222 (1) |
Rotem 2021 |
↑ |
| Diabetes |
4,817/893,519 (2) |
Jo 2019, Kong 2020 |
↑,↑ |
| Fever |
583/95,754 (1) |
Hornig 2018 |
↑ |
| Hyperandrogenemia |
4,022/437,222 (1) |
Rotem 2021 |
↑ |
| Maternal depression |
4,699/709,441 (2) |
Chen 2020, Gao 2015 |
↑,↑ |
| PCOS |
4,022/437,222 (1) |
Rotem 2021 |
↑ |
| Psychiatric disorder |
307/64,754 (1) |
Malm 2016 |
↑ |
↑ indicates significant increased risk association for ASD, ↓ significant decreased risk association, ↔ no significant association.
Biomarkers
Endocrine-disrupting chemicals
One study (Braun et al. 2014) analyzed the effects of endocrine-disrupting chemicals (EDCs) on 175 pregnant individuals, 22 of which had children diagnosed with ASD utilizing semi-Bayesian (β) regression models. Evidence from this study suggests an increased risk of developing ASD when exposed to trans-nonachlor (β 4.1, 95% Confidence Interval [CI]: 0.8, 7.3). A significant decreased risk association was found in children born to individuals with detectable concentrations of β-hexachlorocyclohexane (β -3.3, 95% CI: -6.1, -0.5) or PBDE-85 (β -3.2, 95% CI: -5.9, -0.5).
One study (Ritz et al. 2020) analyzed the risk associations between specific maternal metabolites in the serum of 114 pregnant mothers, 52 of whom had children diagnosed with ASD. The study found decreased risk associations between the metabolite quinoline and ASD. The study utilized partial least square discriminant analysis and found increased risk associations between the metabolites ornithine, 1-methylhistidine, methyl jasmonate, benzoate, nonanioic acid and 10-hydroxydecanoate with ASD diagnosis (statistical quantification not specified). The study also found significant risk associations with seven enriched metabolic pathways utilizing Mummichog pathway analysis, including: glycosphingolipid biosynthesis (p <0.001), phosphatidylinositol phosphate metabolism (p < 0.012), bile acid biosynthesis (p < 0.017), N-Glycan biosynthesis (p < 0.022), glycosphingolipid metabolism (p <0.036), pyrimidine metabolism (p <0.014), and C21-steroid hormone biosynthesis and metabolism (p <0.040).
Methylmercury
One study (McKean et al. 2015) analyzed 257 pregnant individuals exposed to methylmercury during pregnancy by analyzing newborn mercury serum concentrations and creating a toxicokinetic model with information from maternal fish questionnaire. Of these individuals, 164 children were diagnosed with ASD. The article reports that a cumulative exposure to methylmercury does not increase the risk for ASD diagnosis.
Organochlorine pesticides
One study (Lyall et al. 2017) analyzed 1,144 pregnant individuals exposed to organochlorine pesticides during pregnancy of which 545 offspring developed ASD. The study found no increased risk between organochlorine pesticides exposure and ASD.
Organohalogens
One study (Traglia et al. 2017) evaluated the exposure of various organohalogens in 366 children with an ASD diagnosis from a group of 735 total pregnant individuals evaluated. Three polybrominated compounds (PBDE-100, 153, and SUM PBDE) were associated with an increased risk of ASD in the ancestry-adjusted analysis, P < 0.05. Additionally, results supported the concept of genetic control of midgestational biomarkers for environmental exposures by nonoverlapping maternal and fetal genetic determinants, suggesting that the impact of environmental exposures may differ by genetic variation in mothers and/or fetus. Fetal genotypes expressed in placenta can influence maternal physiology and the transplacental transfer of organohalogens.
Organophosphates
Three studies (Millenson et al. 2017; Philippat et al. 2018; Ritz et al. 2020) analyzed exposure of 1,049 individuals to organophosphates during pregnancy of which 64 children were diagnosed with ASD. There was no association found between organophosphate exposure and development of ASD.
Perfluoroalkyls
Two studies analyzed the exposure of 2,165 pregnant individuals to perfluoroalkyls (PFAs) and 773 subsequent ASD diagnoses. Lyall et al. 2018 found a decreased risk association between PFOA (adjusted Odds Ratio [aOR] 0.62, 95% CI: 0.41, 0.93), PFOS (aOR 0.64, 95% CI: 0.43, 0.97) and ASD diagnosis. Liew et al. 2015 found no association between maternal PFA plasma level and autism (Liew et al. 2015). Other studies found increased risk associations between PFOAs and other PFAs (Oh et al. 2021).
Phthalates
Two studies (Oulhote et al. 2020; Shin et al. 2018) evaluated phthalate exposure in 711 pregnant individuals, 48 of whom had children who developed ASD. Oulhoute et al. found significant correlations between increased urinary MBP and MCPP concentrations and SRS score increases of 0.6 (95% CI: 0.1, 1.0) and 0.5 points (95% CI: 0.1, 0.8) respectively, indicating greater social behavior deficiencies. The authors found a decreased risk association in female children between two-fold increases in MEP concentrations and SRS score decreases of -0.4 points (95% CI: -0.7, 0.0). Finally, the authors found significant attenuation of the association between phthalate concentrations and higher SRS scores for DEHP, MCPP and MBP when folic acid was supplemented at ≥400µg/day (p<0.1) (Oulhote et al. 2020). Shin et al. found a significant decreased risk association between the MCPP phthalate in the second trimester (Relative Risk Reduction [RRR] 0.53, 95% CI: 0.32, 0.87) and the development of ASD (Shin et al. 2018). The authors also found a significant decreased risk association for ASD in mothers who took prenatal vitamins and were exposed to MiBP (RRR 0.44, 95% CI: 0.21, 0.88), MCPP (RRR 0.41, 95% CI: 0.20, 0.83), and MCOP (RRR 0.49, 95% CI: 0.27, 0.88) phthalates (Shin et al. 2018). Like Oulhoute et al., other studies also support the correlation between increased phthalates and elevated SRS scores (Patti, Newschaffer, et al. 2021).
Polychlorinated biphenyls
Three studies (Granillo et al. 2019; Lyall et al. 2017; Bernardo et al. 2019) analyzed a total of 2,444 individuals exposed to polychlorinated biphenyls (PCB) during pregnancy of which 574 children were diagnosed with ASD. Only Lyall et al. (2017) found a significant association between PCB exposure and ASD diagnosis (comparing highest versus lowest quartile of PCB 138/158 aOR 1.79, 95% CI: 1.10, 2.71 and PCB 153 aOR 1.82, 95% CI: 1.10, 3.02).
Polyunsaturated fatty acids
One study (Steenweg-de Graaff et al. 2016) reported the exposure of 4,624 pregnancies to polyunsaturated fatty acids and found an increased risk in ASD diagnosis when children were exposed to a lower maternal omega-3: omega-6 ratio (β = 0.009, 95% CI: -0.017, -0.001) as well as higher total omega-6 levels (β~total omega-6~ = 0.11, 95% CI: 0.002, 0.020).
Environmental Exposures
Agricultural pesticides
One study (von Ehrenstein et al. 2019) analyzed the ASD diagnosis of 2,961 children from 38,331 pregnant individuals exposed to agricultural pesticides and found an increased risk associated with pesticide exposure, including chlorpyrifos (Odds Ratio [OR] 1.15, 95% CI: 1.20, 1.29), diazinon (OR 1.14, 95% CI: 1.03, 1.26) and avermectin (OR 1.14, 95% CI: 1.03, 1.26) (von Ehrenstein et al. 2019).
Air conditioning
One study (Gao, Xi, Wu, et al. 2015) analyzed maternal air conditioning use during the pregnancy of 926 individuals, from whom 193 offspring were diagnosed with ASD. Use of air conditioning was found to have a decreased risk association with ASD development (OR 0.32, 95% CI: 0.22 – 0.46) (Gao, Xi, Wu, et al. 2015). The authors hypothesized this could be due to air conditioning correlating with family economic status, season of pregnancy and childbirth as air conditioners are primarily utilized in summer months, or indoor air pollution exposures decreased by air conditioner use.
Asthmagens
Two studies by Singer et al. (2016, 2017) examined the effect of immune-triggering exposures (“asthmagens”) during pregnancy on ASD diagnosis in two separate study populations. A total of 12,332 children from 61,781 pregnant mothers exposed to asthmagens were diagnosed with ASD. The 2016 study (including 463 cases with ASD) found no association between asthmagen exposure and ASD diagnosis. The 2017 more comprehensive study (11,869 cases with ASD) found a decreased risk association between maternal asthmagen exposure and ASD diagnosis (aOR 0.92, 95% CI: 0.86, 0.99).
Caffeine
One study (Patti, Li, et al. 2021) analyzed the intake of caffeine during the two halves of pregnancy in mothers from two different cohorts; 120 mothers from the EARLI birth cohort of which 20 had children diagnosed with ASD and 269 mothers from the HOME cohort of which 43 had children diagnosed with ASD. The study found no significant risk associations between caffeine intake in the EARLI cohort (β per Interquartile Range [IQR] increase [57mg]: 2.0, 95% CI: -0.1, 4.0) or the HOME cohort (β per IQR increase [0.43mg]: 0.6, 95% CI: -0.5, 1.6) and ASD diagnosis by SRS scores. The study did find a significant increased risk association between average caffeine intake and ASD diagnosis in the pooled data analysis (β per IQR increase: 1.2, 95% CI: 0.3, 2.1).
Commercial Pesticides
One study (Schmidt et al. 2017) examined the influence of commercial pesticide exposure, like carbamate and pyrethroid, in 806 pregnancies of which 466 developed ASD. The study found no significant association between commercial pesticide exposure and ASD diagnosis (OR 2.0, 95% CI: 0.9, 4.2).
One study (Windham et al. 2006) analyzed the exposure of 941 pregnant individuals to heavy metals. A total of 284 of the children from the cohort were diagnosed with ASD. The authors found a significant association between ASD diagnosis and heavy metal concentrations in ambient air, with increased risk at higher concentrations (fourth quartile aOR 1.7, 95% CI: 1.0, 3.0 and third quartile aOR 1.95, 95% CI: 1.2, 3.1).
One study (Skogheim et al. 2021) analyzed exposure to arsenic in 1,431 mothers, 397 of which had children diagnosed with ASD. Significant increased risk associations were found between the second quartile of arsenic (OR 1.77, 95% CI: 1.26, 2.49) and the fourth quartiles of cadmium (OR 1.57, 95% CI: 1.07, 2.31) and manganese (OR 1.84, 95% CI: 1.30, 2.59) and ASD diagnosis. The study found significant decreased risk associations between ASD diagnosis and the second quartile of copper (OR 0.69, 95% CI: 0.49, 0.98), fourth quartiles of cesium (OR 0.63, 95% CI: 0.44, 0.91) and zinc (OR 0.63, 95% CI: 0.45, 0.90) and the second, third and fourth quartiles of mercury (OR 0.43-0.56, 95% CI 0.30, 0.80).
High levels of folic acid intake
Two studies (Goodrich et al. 2017; Schmidt et al. 2017) examined the effects of high folic acid intake in 1,412 pregnant individuals. Other studies suggest a decreased risk association between maternal vitamin intake and ASD diagnosis (DeVilbiss et al. 2017). Goodrich et al. examined the influence of high versus low folic acid intake when mothers were exposed to high NO2 levels and found a significant difference in the two populations (NO2 and low FA intake OR 1.53, 95% CI: 0.91, 2.56 versus NO2 and high FA intake OR 0.74, 95% CI: 0.46, 1.19, p-interaction = 0.04) (Goodrich et al. 2017). Schmidt et al. examined the effects of high FA intake combined with exposure to agricultural pesticides and found no significant association with ASD diagnosis (Schmidt et al. 2017). Thus, it appears that high intake of folic acid may be able to attenuate ASD risk from other substances, although the literature reports mixed results.
Indoor pesticide exposure
One study analyzed indoor household pesticide exposure during the pregnancy of 806 individuals (Schmidt et al. 2017). The study compared all groups to a cohort of individuals with low folic acid intake during the first month of pregnancy and no known pesticide exposure. They concluded that the combination of low folic acid intake (≤800 µg) and indoor pesticide exposure led to an increased risk for ASD (aOR 2.5, 95% CI: 1.3, 4.7). There was also a lesser, but still significantly increased risk for ASD among high folic acid intake (≥800 µg) and indoor pesticide use (aOR 1.7, 95% CI: 1.1, 2.8).
Maternal Dental Amalgams
One study (Geier, Kern, and Geier 2009) analyzed the effect of maternal dental amalgams on the development of severe versus mild autism in 100 children. Amalgams leak mercury vapor which poses neurotoxic consequences. However, there is also a possibility of amalgam quantity/exposure correlating with socioeconomic status, dental hygiene, or nutritional intake. These other risks could be considered as controls in future studies. The study found no significant association between the mean number of amalgams and patients with ASD (mild) versus autism (severe) (Geier, Kern, and Geier 2009). However, the study results showed children of mothers with ≥6 amalgams had 3.2 times greater risk association of autism diagnosis compared to mothers with ≤5 (χ2 =6.2, df=1, p=0.0127).
Maternal Fish Consumption
Three studies analyzed maternal fish consumption in 5,345 pregnancies. Gao et al. (2016) reported a significant decreased risk association between fish consumption, particularly grass carp fish, and ASD diagnosis (OR 0.279, 95% CI: 0.095, 0.799). Steenweg-de Gaaf et al. (2016) found no significant association between maternal fish intake and child autistic traits. Vecchione et al. (2020) found an increased risk association between higher fish intake during 21-36 weeks of pregnancy and ASD diagnosis (OR 5.60, 95% CI: 1.03, 17.86).
Maternal Fruit Consumption
One study (Gao et al. 2016) surveyed mothers regarding fruit consumption during pregnancy and found a significant decreased risk association between fruit consumption and ASD diagnosis (OR 0.413, 95% CI: 0.216, 0.804).
Methanol
One study (Walton and Monte 2015) analyzed the effects of maternal methanol consumption in 711 pregnancies and the development of ASD in 161 of the subsequent offspring. There was a significant increased risk association between children who developed ASD having a higher level of maternal methanol exposure during pregnancy (p<0.001).
Air Pollution and Particulate Matter
Two studies analyzed the effects of air pollution on the serum of 214 pregnant mothers, 116 of which had children diagnosed with ASD. Kim et al. (2021) found increased risk associations between the metabolites hypotaurine and urate. The study also found decreased risk associations between the metabolites phenylalanine and 3-hydroxybutanic acid and ASD diagnosis. Goodrich et al. (2017) compared near roadway air pollution of maternal addresses during pregnancy and found no significant association with ASD diagnosis.
Particulate matter (PM) is a type of air pollution composed of various solid particles and liquid components. We identified six studies that analyzed exposure to PM during pregnancy and subsequent ASD diagnosis. A total of 288,721 individuals were exposed to PM during pregnancy with 9,766 children diagnosed with ASD. Three studies found an increased risk of ASD when exposed to PM with diameters ≤2.5µm (PM2.5). Jo et al. (2019) reported a significant increased risk association with exposure during the entire pregnancy (HR 1.17, 95% CI: 1.04, 1.33) and ASD. Raz et al. (2015) reported an increased risk when exposure occurred in the third trimester (OR 1.42 per interquartile range increase in PM2.5, 95% CI: 1.09, 1.86) compared to exposure occurring in the first or second trimester. McGuinn et al. (2020) found significant risk associations between PM2.5 exposure during the third trimester and ASD diagnosis (OR 1.3, 95% CI: 1.0, 1.6). Two other studies found no association between PM exposure and ASD development (Goodrich et al. 2017; Gong et al. 2017). A fifth study (Kalkbrenner et al. 2015) found a significant increased risk association with exposure during the third trimester (aOR 1.36, 95% CI: 1.13, 1.63), but a significant decreased risk association with exposure during the first trimester (aOR 0.86, 95% CI: 0.74, 0.99). Other studies showed an increased risk association between PM exposure in mothers with specific genotypes (Volk et al. 2014).
Nitrogen oxides
Three studies evaluated individuals exposed to nitrogen oxides in traffic-related air pollution during pregnancy. Gong et al. (2017) analyzed 23,373 individuals of whom 5,136 had children with ASD diagnoses. There was no significant association found between nitric oxide exposure and ASD development. Goodrich et al. (2017) analyzed the exposure of 606 individuals to nitrous oxide (N2O) of which 346 developed ASD. Goodrich et al. also concluded there is a significantly increased risk between exposure to N2O in individuals who have low folic acid intake levels (OR 1.53, 95% CI: 0.91, 2.56) vs high folic acid intake levels (OR 0.74, 95% CI: 0.46, 1.19) (p-interaction = 0.04). Jo et al. (2019) studied 246,420 children with 2,471 cases of ASD and did not find any significant associations between nitrogen dioxide and ASD.
Occupational Exposures
One study (Windham et al. 2013) examined 941 pregnant individuals with various occupational exposures, of which 284 children were diagnosed with ASD. Categories of occupational exposure were based on employment listed on birth certificates. This study reported that mothers of children with ASD were twice as likely to have an occupational exposure as those with children without ASD (aOR 2.3, 95% CI: 1.3, 4.2). The occupational exposure categories with elevated odds were among mothers with engine exhaust/combustion products (aOR 12.0, 95% CI 1.5, 108.6) and disinfectants (aOR 4.0; 95% CI 1.4, 12.0).
Outdoor Household Pesticides
One study (Schmidt et al. 2017) analyzed the exposure of 806 pregnant individuals to outdoor household pesticides concurrent with either low or high folic acid intake. They found no association between outdoor household pesticide exposure and ASD diagnosis in either group.
Ozone
Three studies (Goodrich et al. 2018; Jo et al. 2019; McGuinn et al. 2020] analyzed the exposure of 248,555 pregnant individuals to ozone (O3) of which 3,491 had children who developed ASD. Goodrich et al. (2017) and Jo et al. (2019) found no relationship between O3 and ASD diagnosis (aOR 1.14; 95% CI 0.71, 1.82 and adjusted Hazard Ratio [aHR] 1.10, 95% CI 0.95, 1.26, respectively). McGuinn et al. (2020) found a significant increased risk association between ozone exposure during T3 and ASD diagnosis per IQRWidth of 6.6 parts per billion (OR 1.2, 95% CI: 1.1, 1.4).
Solvents
Two studies evaluated the risk of exposure to solvents, such as aromatic and chlorinated solvents, on the risk of ASD diagnosis (McCanlies et al. 2019; Windham et al. 2006). Of the 1,892 pregnant individuals included in these studies, 821 had children were diagnosed with ASD. Windham et al. (2006) found a significant increased risk association between solvent exposure and ASD diagnosis in two different exposure quartiles (third quartile aOR 1.95, 95% CI: 1.2-3.1 and fourth quartile aOR 1.7, 95% CI: 1.0-3.0). McCanlies et al. (2019) also found a significant increased risk association between solvent exposure and ASD diagnosis (OR 1.5, 95% CI: 1.01, 2.23).
Medication Exposures
Antibiotics
One study (Hamad et al. 2019) examined 214,834 pregnant individuals exposed to antibiotics, defined as filling an antibiotic prescription during pregnancy. Of the children born to these individuals, 2,965 were later diagnosed with ASD. The authors reported an increased risk of ASD with antibiotic exposure (aHR 1.10, 95% CI 1.01, 1.19).
Antidepressants
Two studies examined the effects of antidepressant exposure in 1,645,383 pregnancies with 14,924 ASD diagnoses. Malm et al. (2016) found a significant increased risk association between antidepressant exposure, specifically SSRIs, with ASD diagnosis (aHR 1.40, 95% CI: 1.02, 1.92). Sujan et al. (2017) found no association between antidepressant exposure during the first trimester and ASD diagnosis (aHR 0.83, 95% CI: 0.62, 1.13).
Genetic Variability
One study (Hollowood-Jones et al. 2020) analyzed maternal and urine blood samples from 59 mother-child pairs of which 30 children had diagnoses of ASD. The various methylenetetrahydrofolate reductase variants were examined in addition to several biomarkers and metabolites. The study did not find a significant risk association between MTHFR mutation A1298C (p=0.15) or C677T (p=0.90) and ASD. They did find significant increased risk associations between lower vitamin B12 levels (Ratio 0.75, p<0.00), SAM/SAH ratios (Ratio 0.93, p=0.01), 4-vinylphenol sulfate (Ratio 0.31, p<0.00), NAD+ (Ratio 0.41, p=0.03), gamma-glutamylglycine (Ratio 0.27, p<0.00), cinnamoylglycine (Ratio 0.46, p=0.01), propionylglycine (Ratio 0.48, p=0.01), carnitine-conjugated metabolites (Ratios 0.63-0.87, p≤0.03) and ASD diagnoses. The authors also found significant increased risk associations between higher Glu-Cyst (Ratio 1.10, p=0.01), fCysteine (Ratio 1.06, p=0.01), fCystine (Ratio 1.07, p=0.02), dimethyl sulfone (Ratio 18.7, p=0.01) and ASD diagnoses.
Placental Differently Methylated Regions (DMRs)
One study analyzed placental DMRs of 41 pregnant individuals who had 20 children diagnosed with ASD (Zhu et al. 2019). Of 400 potential DMRs, methylation of specific genes differed by up to 10% between the cases with ASD and typically developing controls. Two placental DMRs (CYP2E1 and IRS2) were found to be potentially early epigenetic markers in the placenta for ASD. DMRs were also enriched around placental H3K4me3 and brain H3K4me3 which regulate gene functions (von Ehrenstein et al. 2020).
Paraoxonase [PON1] genotype
One study examined 224 mother-child pairs of which 6 children were diagnosed with ASD (Millenson et al. 2017). PON1 genotypes in cord blood were analyzed to determine their genetic impact on the association between second trimester urine organophosphate concentrations and ASD diagnoses. The PON genotype did not modify the association between prenatal urinary organophosphate concentrations and the social behaviors of children.
Maternal Illnesses and Conditions
One study (Rotem et al. 2020) included 437,222 mother-child pairs of which 4,022 children were diagnosed with ASD. The authors report significant increased risk associations between individuals diagnosed preconceptionally with PCOS and subsequent ASD diagnosis (OR 1.42, 95% CI: 1.24, 1.64). The authors found a significant increased risk association between ASD diagnosis and mothers with any condition of hyperandrogenemia (OR 1.41, 95% CI: 1.10, 1.79), hirsutism (OR 1.30, 95% CI: 1.10, 1.53), acne (OR 1.16, 95% CI: 1.08, 1.25) and infertility (OR 1.19, 95% CI: 1.10, 1.28).
Diabetes
Two studies included 893,519 mother-child pairs, of whom 4,817 children were diagnosed with ASD. Jo et al. (2019) reported an increased risk of ASD diagnosis in children of those mothers with pre-existing type 2 diabetes (HR 1.60, 95% CI 1.26, 2.16). Kong et al. (2020) reported a significant risk association between DM2 in severely obese individuals and subsequent diagnosis of ASD (HR 2.28, 95% CI: 1.18, 4.41). Kong et al. also reported a significant increased risk association between insulin-treated pregestational diabetes in obese individuals (HR 3.61, 95% CI: 1.61, 8.07) severely obese individuals (HR 5.93, 95% CI: 2.81, 12.52) and ASD diagnosis. Children of mothers with gestational diabetes mellitus at fewer than 24 weeks gestational age were found to be at an increased risk of ASD when exposed to higher levels of residential ozone O3 throughout pregnancy (HR 1.50, 95% CI 1.08, 2.09). Kong et al. found a significant increased risk association between gestational diabetes in overweight individuals (HR 1.28, 95% CI: 1.07, 1.53) and obese individuals (HR 1.57, 95% CI: 1.26, 1.95) and subsequent ASD diagnosis. Other studies also support the findings of an increased risk between gestational diabetes and ASD diagnosis (Li et al. 2021).
Fever
One study (Hornig et al. 2017) examined 95,754 pregnant individuals with reports on fevers during pregnancy. 583 children from this cohort developed ASD. The study reports maternal fever in the second trimester, regardless of antipyretic use, was associated with an increased risk of ASD diagnosis (aOR 1.40, 95% CI 1.11, 1.77).
Maternal depression
Two studies analyzed the presence of maternal depression in 709,441 pregnant individuals of whom 4,699 had children that developed ASD (Gao, Xi, Wu, et al. 2015; Chen et al. 2020). Gao et al. and Chen et al. both found an increased risk in ASD diagnosis in children born to mothers with maternal depression during pregnancy (OR 4.82, 95% CI: 3.08, 7.63 and HR 1.58, 95% CI: 1.11, 2.25, respectively). Neither study examined whether the depression was treated. Chen et al. also found a significant risk association between maternal depression diagnosed before pregnancy (HR 2.01, 95% CI: 1.70, 2.37) and subsequent diagnoses of ASD.
Psychiatric disorder
One study (Malm et al. 2016) examined the diagnosis of a psychiatric disorder in 64,754 pregnant individuals of whom 307 children developed ASD. These diagnoses included depression, anxiety, bipolar disorder, and undefined psychosis. The study found an increased risk of ASD development in children of mothers with psychiatric disorder diagnoses who were not on antidepressants during pregnancy (aHR 1.59, 95% CI: 1.16, 2.18). There was also an increased risk of ASD in pregnancies exposed to SSRIs, as detailed in medication exposure section above.
This descriptive systematic review found that several pregnancy exposures and biomarkers may be associated with ASD in offspring. In this systematic review, we assessed 46 exposures across 47 articles. There were exposures with significant, but mostly small, increased or decreased risk associations across all our classification categories [biomarkers, environmental exposures, medication exposures, genetic variability, and maternal illnesses and conditions]. The risk and protective associations are concisely depicted in Table 2.
Our work complements the work of meta-analyses published in 2009 and 2017, an umbrella review published in 2019, and a systematic review of biomarkers published in 2019 (Gardener, Spiegelman, and Buka 2009; Frye et al. 2019; Wang et al. 2017). These studies investigate the prenatal, perinatal, and postnatal risk factors for child autism and report inconsistent conclusions regarding exposures associated with autism. Our results complement some findings but update and are different from some other findings from these studies. The 2017 meta-analyses collated data from 17 studies published between 2002 and 2016 (Wang et al. 2017). The 2019 umbrella reviewed studies published between 2012-2018 that evaluated biomarkers and environmental risk factors related to autism and completed credibility assessments on these studies to quantitatively evaluate their levels of significant (J. Y. Kim et al. 2019). The biomarker review categorized biomarkers into the natural historical timeline of ASD including prenatal, pre-symptomatic, diagnostic, subgrouping, and treatment. Consistent with our study findings, these studies reported that factors associated with increased risk of autism include maternal metabolic syndromes like diabetes and fetal distress, and a factor that was not associated with an increased risk of autism, the presence of a nuchal cord. Using different inclusion criteria from our study results, others included labor characteristics and reported an increased risk of autism with breech presentations, gestational age ≤36 weeks, cesarean delivery, fetal distress, and pre-pregnancy antidepressant use, which our study found to be not significantly related with autism (Gardener, Spiegelman, and Buka 2009; Frye et al. 2019; Wang et al. 2017; J. Y. Kim et al. 2019).
Throughout our analysis of environmental exposures related to ASD, there tended to be some contradictory results about the risk associated with several environmental exposures including particulate matter, nitrous oxide, solvents, and maternal fish consumption. There are several possible explanations for the varied results found in these factors. Some environmental exposures have unmeasurable variations in exposure levels and also are complicated by confounding exposures. These potential confounders may not have been measured in the same way across different studies. Assessing the association of maternal fish consumption and ASD, for example, is complicated by the varied levels of healthy nutrients and harmful contaminates present in each fish. Fish can contain varying quantities of protein, long-chain polyunsaturated fatty acids, iodine, selenium, and vitamin D (healthy nutrients in moderation), in addition to mercury, arsenide, dioxin, and polychlorinated biphenyls (potential toxins). Without controlling the specific quantity of each component in the fish, the precise risk associated with fish consumption is not able to be precisely evaluated. The association between fish consumption and ASD is further complicated by epigenetic variability in prenatal exposure to developmental toxins like mercury and arsenic (Liew et al. 2015).
Epigenetic modifications may modify in-utero response to environmental toxins and nutrients.
In our review, several studies examined potential genetic components of ASD risks. Millenson et al. (2017) evaluated cord blood PON genotypes related to organophosphate concentrations and ASD. They hypothesized that because enzyme polymorphisms effect metabolism of exposures, ASD risk associated with specific exposures metabolized by these enzymes could be impacted by various genotypes of enzyme functioning. Zhu et al. (2019) measured placental DMRs and found 400 differently methylated regions accounting for 596 genes between placentas of children with ASD and children who were typically developing. These findings suggest that epigenetics could have a significant role in ASD outcomes, and that a compounded effect of genetic components combined with exposures could be related to these outcomes. Two of the studies in our review reported evidence in support of this theory. Traglia et al. (2017) and Lyall et al. (2017, 2018) reported more significant exposure risk associations in the ancestry-adjusted analyses of their exposures than in the analyses that did not account for ancestry genetics. There is also some literature suggesting that maternal smoking status, BMI, and antibiotic use during pregnancy can also impact gene regulation in newborns. This suggests that not only inherent genetic variations, but also other acquired epigenetic variations, may play a role in the development of ASD (Nye et al. 2014; Kile et al. 2012). These findings support the need for further work that examines compounded risks related to ASD, especially accounting for epigenetic variations.
According to the developmental origins of disease hypothesis, fetal development, physiology, metabolism, and programming may be altered by maternal nutritional and endocrine status. This embryonic developmental period is theorized to predispose fetuses to diseases even through adult life, including cardiovascular, metabolic, neurologic, and endocrine-related diseases (Nye et al. 2014). The pathophysiology behind this process may be through altered epigenetic programming that occurs because of embryonic exposures. Genetic inheritance of gene regulatory mechanisms is theorized to contribute to differences in circulating levels and processing of embryonic exposures (Traglia et al. 2017). As genetic contributions predispose fetuses to varying levels of circulating exposures during the embryonic developmental period, fetuses may then experience differential risks for developmental outcomes related to these exposures.
To minimize confounding variables in analyzing compounded exposure and factors related to autism, biomarkers are an objectively useful indicator. As defined by the World Health Organization, a biomarker is a chemical, its metabolite, or the product of an interaction between a chemical and target that is measured in the human body (Katherine and Shea 2011). Because biomarkers can be objective measurements, they provide insight into physiologic and pathophysiological processes and pharmacologic responses. Their objective specificity also supports their potential use as predictive and prognostic indicators. In our review, we analyzed biomarkers related to environmental exposures and nutritional intake. In the literature, other suggested potential biomarkers related to ASD include maternal IgG antibodies, amino acid and acyl-carnitine metabolites, and protein levels (Frye et al. 2019).
Most of the significant associations we encountered were mild, with odds ratios just above or below 1.0. A few biomarkers had stronger associations, such as: endocrine-disrupting chemicals (ORs 4.1, -3.3, and -3.2), some phthalates (ORs 0.44, 0.41, and 0.49), polyunsaturated fats (βs 0.009 and 0.11), using air conditioning (OR 0.32), indoor pesticide exposure in low folate intake women (OR 2.5), having ≥6 dental amalgams (OR 3.2), maternal fish and fruit consumption (OR 0.28 and 0.41, respectively), having newborn complications (OR 4.7), occupational exposures (OR 2.3), and maternal depression (OR 4.82). These exposures in particular call for further investigation and replication. Many of them may have roots in social determinants of health and the environment. Further research examining both maternal and newborn biomarkers in relation to ASD could progress the effort to identify predictive or prognostic biomarkers.
Developing a predictive model for ASD could be useful clinically. As Hollowood-Jones et al. demonstrated, models with clusters of multiple metabolites could create more specificity and sensitivity in predicting ASD (Hollowood-Jones et al. 2020). Understanding that a newborn may be at high risk given pregnancy exposures and potentially measuring predictive markers in cord blood could allow for new parents to be educated regarding resources and therapies that could optimize their newborn’s development. Additionally, requests for optimizing prenatal supplements to improve newborn outcomes have been published (Adams et al. 2021, 2022).
Our study is limited in that the array of different exposure biomarkers did not allow for a quantitative synthesis. The studies had valid findings internally, but the diversity of measured exposures and covariates in models precluded any meta-analysis. We attempted in Table 2 to systematically synthesize the data reviewed in a graphic way to guide other researchers and those analyzing exposures. Utilizing these various exposures and measures could be combined using machine learning techniques to build a predictive algorithm that could be independently tested in a cohort. This could then be coupled to trials of early childhood interventions aimed at optimizing childhood development. Additionally, educating pregnant individuals and families on pregnancy exposures that could reduce the risk of ASD might inspire other healthy behavior changes.
Our study has several strengths that contribute to the current published literature surrounding this subject. First, it includes articles published through 2021, adding new studies which were not present in other reviews. Additionally, we attempted to be more comprehensive in our approach to predictive exposures, including a wide array of exposure categories to assimilate a comprehensive overview of risk factors. As such, we were able to characterize several factors that were not included in other reviews. Organizing these exposures by category could allow for researchers to identify and potentially build models for upcoming analyses and trials.
In conclusion, several pregnancy exposures and biomarkers are associated with the development of ASD in children. Continued work to build combined models to allow for determination of the strongest associated modifiable factors is important to unlocking mechanisms and potentially protective measures for families. As ASD can have lifelong impact on an individual and family, better understanding its risks and being able to provide resources for families is crucial to optimal health.
HIGHLIGHTS
-
Ovid MEDLINE systematic review 2006 – 2021 of maternal factors associated with autism
-
47 studies with exposures grouped as biomarkers, exposures, and genetic variability
-
Some exposures with persistently increased risk of autism; others with decreased risk
-
Genetic mutations may play role in impact of exposures’ risk of autism
ACKNOWLEDGEMENTS
Thanks to Dr. Joanne K Daggya for assistance with interpretation of statistical analyses.
aDepartment of Biostatistics and Health Data Science, Indiana University School of Medicine, 550 N. University Blvd., Suite 2301, Indianapolis, IN, USA 46202
CONFLICTS OF INTEREST
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: James Adams reports a relationship with HealthyBaby that includes: equity or stocks. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
SOURCE OF FUNDING
This report did not receive any financial support and is not affiliated with any organization.
Preliminary results were reported via poster presentation at ACOG 2021 Annual Clinical and Scientific Meeting virtually April 30 – May 2, 2021.
CORRESPONDING AUTHOR
Shae N. Jansen; Department of Obstetrics and Gynecology, Indiana University School of Medicine, 550 N. University Blvd., Suite 2301, Indianapolis, IN, USA 46202; Cell phone – (317) 694-8210; email address: shjansen@iu.edu.